“Botanicals” = plants
Process and enzyme mix is proprietary and determined by starting material. Currently, extracting plants for cannabinoids, terpenes, and acidic forms. Applicable to pharmaceutical, nutraceutical, wellness, beverage, and cannabis markets.
Absolutely.
Except in cannabis. Where “botanicals” are everything except cannabis.
Why not mention which plants? Is that proprietary too?
You chose “botanicals” to obsfucate or to “go with the lingo”…In which case you used it wrong.
(Totally agree that the way the cannabis industry uses “botanicals” is horse shit… but that’s the field you chose to play on)
The published route of using cellulases and pectinases doesn’t make sense based on the biology. IMO.
Why bother posting if you’re not willing to discuss that concept?
You know, either explain why I’m wrong, or explain what you’re doing differently.
If you aren’t willing to state which compartment(s) you’re attempting to open then you’re not gonna gain a whole lot of traction here.
What portion of cannabinoids in your material do you believe to be intra-cellular?
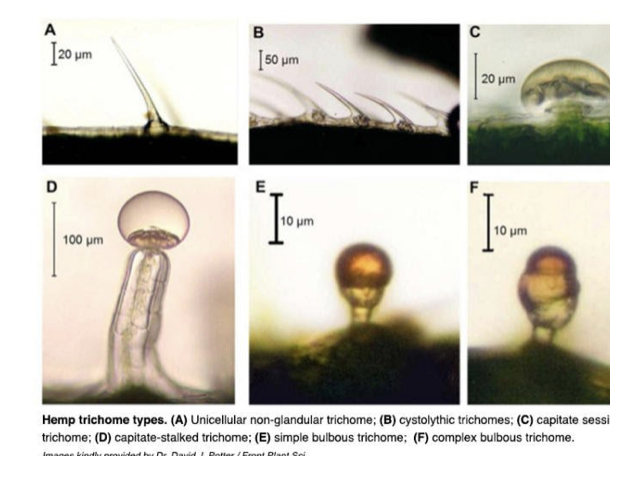
The primary “this works in cannabis” seems to be this ref…
Kitrytė V., Bagdonaitė D., Venskutonis P.R. Biorefining of industrial hemp (Cannabis sativa L.) threshing residues into cannabinoid and antioxidant fractions by supercritical carbon dioxide, pressurized liquid and enzyme-assisted extractions. Food Chem. 2018;267:420–429. doi: 10.1016/j.foodchem.2017.09.080.
I’ll absolutely give you that extracting cannabinoids from fiber type hemp threshing residues might work better than more traditional methods.
Not sure why anyone in their right mind would pursue that route though…
This appears to be another case of “unclear on industry specific jargon, but using it anyway”.
I assume you mean “acidic cannabinoids”…which is covered by “cannabinoids”.
“Cannabinoids, both neutral and acidic” demonstrates an understanding rather than a mere wish to “use all the words”.
If you are unwilling give even minimal information on how or why, are you able to provide “how much”?
ie a break down on equipment cost, consumables, yields, total production cost on finished goods?
Is this an AI list…or do you really have expertise here?
If you do…well you might be the perfect person to decipher the extraction method used to produce the crystal in the THCA X-ray diffraction study:
It was done under very strange circumstances…
Take a look at the methodology…Low pressure CO2, 1000psi for a few hours .
the yield was only about 2% of the total cannabinoic, but they obtained pure crystalline THCA (R-COOH) in the evaporation chamber as a crystal…
As if they selected the polarity to only extract R-COOH dimers…
You are the only person I have seen on this site even mention the Acidity of CO2 liquid under pressure…so I hoping you can shed some light on this rather bizarre extraction of THCA, cannabinoic. I wrote the crystallographer at the time, but he was only handed the material in crystalline form, and the other group that actually preformed the extract would not respond to query.
I should like to further categorize your classification of “Cannabinoids , both neutral and acidic. In the latter form “Acidic” …we should point out that the Acidic comes in two forms RCOOH and RCOO- , which in redundant terminology are referred to as “neutral” and “anionic” forms of the “acidic”.
Which of these two forms you are dealing with depends solvent environment. And further more both the “neutral” and anionic forms have intramolecular h-bonded hexagonal ring forms involving the carboxyl moiety and the phenolic hydrogen. Ones choice of solvent environment , whether non-polar, polar aprotic or polar protic, totally controls the equilibrium distribution of these “acidic” forms in dilute solution. In concentrated solutions approaching tens to hundreds of millimolar (aka concentrates/mother liquids) dimer formation should be seriously considered as well depending on solvent …the later includes dimers with homologous base as well. The intramolecular h-bonded ring of the anionic form gives rise to rather high P values when looking a partition quotient values vs pH of solution in polar protic solution. Also in conditions where one studies pKa variation vs solvent choice….especially in polar aprotic and non polar solvents, one sees remarkable variation of form and solubilities of the cannabinoics due to high pKa values in alternative solvents.
Here is a wake up call for 4200 Water and Ethanol extractors i.e., polar protic: Ultrasonic-Assisted Extraction of Cannabidiolic Acid from Cannabis Biomass
“
Heresy:
“An alternative method designed to increase solubility is ultrasonic-assisted extraction. In this protocol, the impact of solvent polarity (acetonitrile 0.46, ethanol 0.65, methanol 0.76, and water 1.00) and concentration (20%, 50%, 70%, 90%, and 100%) on ultrasonic-assisted extraction efficiency has been examined. Results show that water was the least effective and acetonitrile was the most effective solvent examined. Ethanol was further examined since it has the lowest toxicity and is generally regarded as safe (GRAS). Surprisingly, 50% ethanol in water is the most effective ethanol concentration for extracting the highest amount of cannabinoids from hemp”
Does anyone think anions have anything to do with these observations?
I should like to stress that these results are reported for Hemp…so it is highly likely that the yields reported refer to CBDA not THCA.
I heard it helps to get salty with it too…
I have a tube of material sitting in salt water as we note.
I cannot reproduce the “standard tea” experiment discussed…
But I’m using bubble…dried. Not harvest biomass.
Thanks for joining the conversation, I’ll reach out for more information
Yes, this is well established and no one is arguing the merit of traditional extraction practices. Hydrocarbon is king for the resin boys. Rosin is king for the hash heads.
Ethanol is great for scaled distillate production in the current landscape. Right now,
I am interested in using enzymes to break down undesirable compounds from “finished” products. Hopefully someone with experience like @rawzyn can help.
In the future, enzymes may be very interesting. I would be careful challenging the economics and efficiency of nature’s extractor. I certainly don’t know how to use them… but someone does. The industry has a long way to mature before you really have to worry about it in my opinion- but once legal red tape is cut, interstate(or international) supply chains well established… all the sudden that 10000+kg/hr throughput becomes necessary again and everyone rushes back to find scalable solutions.
Ultrasonics introduce a whole new world of issues. Its very poorly understood, except by a handful of professors in Spain(I don’t know why Spain), and a few scattered in Russia, Germany and US(German and Russian immigrants ![]() ). There is a huge disconnect between those who deeply understand ultrasonics and those who see the application for them. Your wake up call should be a warning… its a black hole. I’m well past the event horizon and don’t advise following.
). There is a huge disconnect between those who deeply understand ultrasonics and those who see the application for them. Your wake up call should be a warning… its a black hole. I’m well past the event horizon and don’t advise following.
Also, I can’t read that paper due to paywall.
Are you implying deprotonated water extraction assisted by ultrasonics?
Enzyme assisted post-processing isn’t a horrible idea…especially if you are looking to achieve simple things where nature has already provided a solution or model (eg modding rubisco)
We have been “evolving” enzymes for use as bio-catalysts for many years.
…and have recently cracked the code for determining the folding of proteins from their primary sequence (alphafold2)
So picking a problem, then designing an enzyme to solve it, will be the right way to perform a whole heap of things in the not too distant future.
Not convinced that extracting cannabinoids or terpenes from the compartment they were presented to us in is gonna be one of those.
Unless enzyme-assisted cleanup/whatever is cheap cheap cheap, it’s going to have a really hard time competing with traditional methods.
About the only place I feel it might be economically useful is for pesticide remediation? If that’s even theoretically possible.
some of us with more “bench chem” and product isolation background have been using “ultrasonics on Cannabis biomass” for ten years or more. The essence of that paper is the water/ethanol ratio…not necessarily the ultrasonic assist.
“Are you implying deprotonated water extraction assisted by ultrasonics?” It would seem the entire subject matter is beyond your horizon…at present.
Look, people have made millions extracting marijuana with butane. If that is what you are doing…just keep at it…milk that cow. Do I have any advice about you doing Enzymatic treatment of “finished” cannabinoid products in Aqueous polar protic solutions? Well considering that question alone should be a sufficient answer….at present time.
Given your curiosity potential as a driving force as opposed to dollars…if high enough …you might find something.
Currently the bho industry state of the art …is high purity THCA products. Large scale and almost unbelievable purity. If you are at this stage in technology…I have to ask why would you want to do “Enzymatic clean up”…you would only be contaminating the product with proteins, cofactors, buffers/water and detergent…and above all, what chemical conversion do you have in mind, that you want a catalyst?
Right grad student and some compute could probably solve some of them now…
Our machine overlords should be up for it on their own relatively soon with appropriate training.
Wild times we’ve chosen to experience…
Unfortunately if someone wants to do such a thing on any kind of reasonable timescale, and doesn’t have access to a local supercomputer, the closest thing to the correct tool for the job looks a lot like ticking every one of the most expensive boxes on this page. $350k-$500k is not an unreasonable guess for a starting point.
At the very least, the above should let you narrow down where the correct range of answers may lie before you need to start spending serious money on your computing capabilities.
And yes, it IS possible to rent compute in the cloud. But remember, the cloud is just someone else’s computer. Magic dirt that eats pesticides sure seems like the kind of thing that’s worth keeping under your own hat until you want to let it out.
… Sure makes me wish I’d taken that fully funded masters in artificial intelligence back in 2016.
The original idea for Enzyme assisted extraction, at least when I originally brought it up, was to break down trichome walls. I was sort of envisioning a ‘French press’ style extraction where you would use water and enzyme to prep biomass, use sonication to separate product from biomass, then press the biomass to the bottom and decant product from the surface of the water.
At the time Carbon Chemistry had just rolled out degumming enzyme and I was searching for as many uses of the product as possible. It was my hope that there were better ways to use water based extraction, outside of trying to knock trichomes off the biomass and then press them.
https://doi.org/10.1016/j.biortech.2011.01.013
Enzyme assisted dumpster fires anyone?!?
That is certainly targeting the correct compartment.
Although I’m reasonably sure that detergents alone would work equally well to kick that particular door in, without adding the additional complexity and expense of removing the enzymes.
Nailing the enzymes to a solid support, and/or modding their active site so they simply grab your target of choice till you elute them is another reasonable approach
Thanks for the book recommendation! I agree to all your points.
The “Isn’t Horrible” is how I got to enzymes to begin with. There are many ways to accomplish the same goal.
My question was based on your post/divergent reply. Insulting me doesn’t make you look better. Clearly, you are an intelligent chemist who could contribute beyond belittling strangers. I don’t understand where the hostility is coming from. Want a dab?
Not what I was asking or thinking about. YOU introduced polar protic solutions, ultrasonics, ethanol/water ratios, etc into the conversion about enzymes. I agree on EAE for crude biomass production is far from economical right now and immediate future.
Or I can reach out the community and pay consultants, share information with other members, and help the industry improve. I really like what the future forums stand for. That sort of toxic business approach won’t get you far. Maybe if you know so much, you could offer consulting and make $?
Getting back to topic…
Thanks for joining in Shadownaught, who better to reach out to. ![]()
Have you guys found your enzyme to target only phospholipids? Have you worked on any enzyme post-processing or downstream purification?
Nailed it. This is essentially where I’m at right now. Going through some old threads tells me other people went down the enzyme rabbit hole. Aside from the physical difficulty of removing enzymes from solution(could be automated at scale so I’m not worried). Modding enzyme active sites is outside my wheelhouse but necessary for my project, hence my search for consulting.
Back in 2000 when working in the Brenner lab at UCB, one of my primary functions was scavenging discarded desktops and tying them into compute clusters while we built out our own “super computer”.
The suitability of GPU vs CPU for this particular problem was obvious but not yet being actively exploited at that point
Pretty sure the room full of commodity hardware we eventually assembled to address protein folding could be outpaced by a single high-end Mac these days.
Or possibly even a medium grade cellphone.
Yes, I did. There is one patent concerning Enzyme application for cannabis raw material…I think it was originally by a Swiss company…but now there is a USA branch…anyways I read the patent in detail some years ago……Seemed like an odd approach. There was a guy from USA trying to implement this process in Africa on a large scale. Is it you?
My recollection of the details of the first step of the patent was in fact of an enzyme treatment of biomass in Aqueous (polar Protic ) solution. (Perhaps with EtOH…can not remember). So not knowing of any other literature of the processing of Marijuana biomass with “Enzymes” , I just assumed you are talking about this process…
There is one reference to THCA synthase working in non polar extracts of marijuana buds. But if I remember correctly it was just a comment. If you look back in “butane works no theory” I posted a comment about it years ago.
I have no idea what you are Thinking about …but I am not sure you have a good handle on the solution -chemistry of Cannabinoids in “polar protic” solvents. I’ll gladly remove the WTF comment. But I think you need a little primer on polar protic…cannabinoid solutions. and as far as enzymes go I do not know of any natural enzymes not assembled in polar protic environment.(excepting some rare examples of enzymes being prepared by solid phase techniques) So if you don’t see my “divergent” connection, I apologize….but your comments about alcohol water systems and anions…seemed as if you were speaking of personal knowledge base
Can you further clarify your advice? Please advise.
I really appreciate your clarifications. Perhaps I need to smoke as well- I was not clear and defensive. I am not a Chemist, I am an Engineer, so I may have come across redundant when I asked for clarification.
I heard of the Swiss company with enzymatic extraction. I am not them, nor have any affiliation aside from “knowing of them.” I believe there is a thread about them floating around. I was certainly referencing them in my initial post. They were the first I heard of using EAE, however I recently heard rumors(whatever they are worth) of other large operations stateside who are working with EAE. I don’t know for sure, otherwise I’d share the company names.
I’d love to and maybe we can resurrect an ultrasonics thread and really start getting into it. #MakeFutureGreatAgain?
I have done R&D using ultrasonics for the better part of a decade. Ultimately, 90% of the application found in Cannabis is 20-40khz transducer probes used for nano. A few try to use them for extraction, but find the yield gains very small and the cleanup cost on post too high. I’m sure no one here needs convincing. However- this is barely scratching the surface. Ultrasonics range from, 20khz-2mhz. There are very interesting phenomenon at play such as: resonant/natural frequency of different compounds, whether crystalline/amorphous, distribution of power, field-exposure time, field intensity, secondary harmonics, transfer medium, etc. that many don’t consider. As you move up frequency, new phenomenon occur. When you get into megasonics(>2mhz), chemical changes are far more likely to occur. This leads me to my warning: There are often unintended consequences with ultrasonics. On the surface, using sound seems intuitively safe… however you can really change the chemistry of your solution. There is undoubtedly the effects of thermal decomposition, polymerization after extended exposure, and hydrolysis is far more likely to occur in many materials. Rosin extractors quickly moved away from Ultrasonic curing when they discovered profile changes in their product. Moreover, operator safety should really be considered. These sonicators can be REALLY loud(think fighter jet at full throttle loud). Even a little unit(~100w) can really hurt you and those sound enclosure provided by fisher don’t cut it. Aside from unknown effects, loud operation, there is the whole probe shedding macro particles of titanium oxide into your solution in direct application, and the high cost of getting into ultrasonics. Also, lead from PZT elements, vessel pitting, PTFE offgassing… All of these challenges can be designed around($$$$) but often overlooked. Like I said, black hole.