You get the chlorophyll out with carbon. It never even gets to the glass for me. From the pot it gets filtered out immediately after extraction. Also you can use uv light to break down chlorophyll if necessary.
I just realized I totally forgot to post my end product after distillation here after running through the column. Went from this to this with one run on the short path. Has a bit of green because I switched to mains a bit early, but it was going to isolate anyway so I didn’t bother re-running. 88% CBD, 95% total. Heads didn’t have too much green, but enough to be slightly visible contaminating the body. The first pic is diluted in 4:1 heptane, and ended up getting even darker once all of it finally came through the column.
Looks great
we use a hotplate with overhead stirrer to control the heat. we’ve been heating Etoh/crude slurry and adding adsorbants pre filtration.
Here are the tests for the water clear disty I got above. Turns out I’m getting a good amount of isomerization
I was afraid of that ? To bad heads up and full force ahead ![]()
![]()
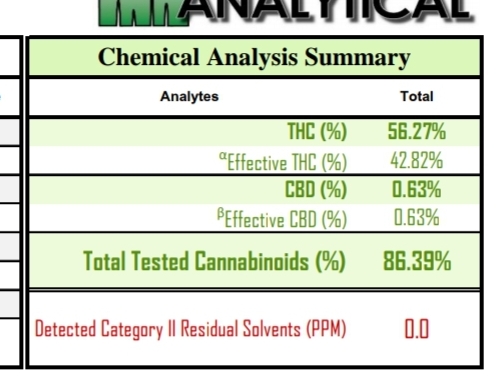
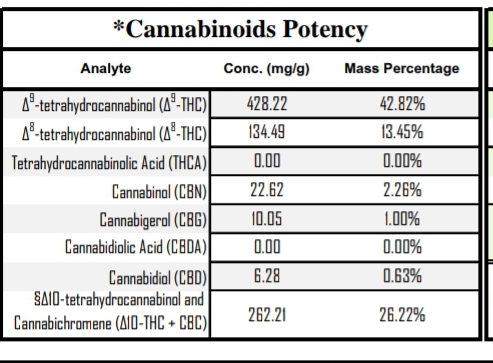
D10 and CBC together? I wonder why they show that analyte that way?
Wow that is a weaksauce test result. They can’t separate d10 and CBC, nor have a standard for the former, so they are just returning a dual result – ignoring that the response factor for the two is likely different, thus destroying all validity in the number.
'cause that’s the best they can manage with HPLC DAD?!?
indeed @MagisterChemist: marginally better than just calling it all CBC I guess…because it’s presumably mostly not CBC.
Except that the problem is the calibration curve was made using CBC. For all we know 10% d10 makes a peak the size of 20% CBC. So you could have a sample with 5% d10 and 5% cbc and it would show up as 15% “d10 and CBC” - a completely wrong answer. On DAD, you cannot quantify something without knowing it’s response factor/making a unique calibration.
I guess we do now… ![]()
my understanding was that if the retention time is the same, and the absorbance maximum is the same, then even having a standard for both doesn’t make them resolvable via hplc.
I imagine having standards for both might allow one to tweak their HPLC solvent system or stationary phase to achieve separation, but I don’t understand how it would help if they’re running on top of each other and absorbing at the same frequency.
edit: Doh! ![]()
I read that as “For we all know…” which is totally different.
No, you’re right, as long as they co-retain, they can’t resolve them. The absorbance maximum being the same is also not known (in fact I would guess it’s false considering the very different conjugation of the two). However, if we knew the response factors for the two were equal, we could give a combined percent for the two like they’re trying to do. But they don’t know that they are equal, so they can’t give a real number. If it turns out they aren’t equal, then we can’t even give a combined percent, because the peak size would no longer have a 1:1 correspondence with concentration.
Just some workflow pics out of my experiment log for my upcoming run. I’ll be posting a final log after I get a product I like with a clear SOP.
Sorry they’re sideways. Oh, and a really rough sketch of my filter and recovery layout. The vacuum chamber in the filtration stage is actually just to give me a vacuum gauge in line.
For anyone curious, a vacuum chamber paired with a magnetic stirrer and set in a warm water bath followed by a cold trap is an effective recovery apparatus.
I definitely need to dial in my temperatures a bit more. I used 20 lbs of dry ice to recover 1.75L and that seems a little excessive. I’m maintaining temps by adding dry ice directly to my isopropyl cooling baths and by swapping cooled warm water baths with freshly heated water. Temps were between -20 - 0°C on average in the cooling baths, and ranged between 30 - 60°C for the warm water bath. Internal temperature of the vacuum chamber ranged from 20 - 46°C. Vacuum started at -27inHg and settled at -25inHg. Interior cooling bath was 1L, exterior cooling bath was 3L, warm water bath was 2L. I couldn’t maintain a stronger vacuum because it was making my pump sound unhealthy. Any thoughts or advice?
P. S. I noted an unusual phenomenon. Where the solution wouldn’t bubble when the stir bar decoupled from the stirrer magnet it would boil once the bar was recoupled to the magnet, even when the magnet was not being turned. Just something of note.
Ha I built the same thing but w a coil
Like an empty condensing coil inside of a container you fill with your cooling bath mixture? Or the standard coil of cooling liquid inside of an empty chamber for condensing?
I had originally been playing with the idea of using a coil submerged in a cooling bath that would have an ethanol vapor stream going in and a liquid stream of ethanol coming out the other end, but it’d been suggested by a fellow member that might be overkill.
Purge pot in hot water bath mag stir under beaker inside pot line going to coil inside cooler w modified ac unit for bath coil to another purge pot 2nd purge pot to vac pump I didn’t think of a small coil inside a purge chamber I think more cool fluid around the vapors vs the vapors around the cooling coils will do a more stand up job
Modified ac unit for cooling? I tried to find some information online, but couldn’t get anything. Care to elaborate?
It occurred to me before starting today using small chunks to keep things cold is only increasing the rate of evaporation of the dry ice by increasing surface area. I’ll be trying large chunks today to see if that helps reduce usage. I’ve also added towels for insulation, better than nothing. I still wonder if I’m running either too warm or with too much agitation for how cold my cooking liquid is. I need to find some good formulas on heat transfer from phase change and a few other things to better dial this in.
Also, if it is possible to reduce the amount of solvent to filter by recovering a portion of it without damaging the quality of the oil I highly recommend it. Filtration through a thick cake of t-5 with vacuum is extremely slow. 6hrs or something to filter about 4L.
AC chiller thread ![]() cold for cheap!
cold for cheap!
YouTube ac unit chiller shows it all works great for that amount





