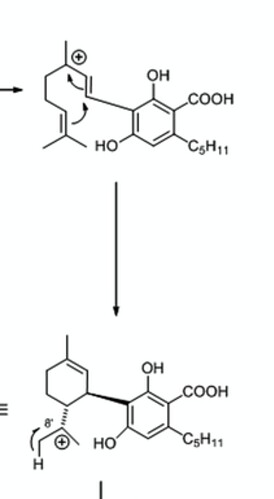
You do realize that the double bond shifts to the 9th position from the tenth position when the enzyme closes the ring on top. There is no way to close the ring and shift it to the 8th position from the 10th. You wouldnt be able to close the ring.
The plant doesnt make cbn… cbn is formed through oxidation… which is what ive been saying about d8 is the ENVIROMENT makes it not the plant
Hhc im not sure about im going to go through the colorado chromatography lab paper and see if they give a plausable synthesis route
I bet you its not made by the plant though
that’s how we get D9…
…and from there, if we’re not careful with our acid catalyzed reaction, we get D8. no?
if the enzymes function was to make D9, one might expect evolved constraints to prevent that cascade.
What if those constraints were not there (why would they be selected for) in the ancestral enzyme (CBDAS)?
it’s a hypothesis. one that fits @Dr_Jebril’s data better than your alternative. doesn’t make it right. does seem like it’s testable.
plants with only CBDAS are making their D9 with different tooling than those using THCAS…you’re absolutely certain it’s not that tooling that is giving a different D8/D9 ratio?
WHY?!?
certainly looks like the most parsimonious explanation to me…
No thats how we get cbd also.
In BOTH synthesis the ring closes and the double bond shifts to the 9th position. This is only possible because of where the oxygen attacks… if it were to attack on the other side (which would be needed to make your d8) you wouldnt be able to form the ring
Its not different tooling its done the same way there’s only one way to make d9 from its precursor which is the same precursor used to make cbd
two non-identical enzymes, one derived from the other. most certainly “different tooling”.
same feed stock. different results. both enzymes CAN make CBD, CBC, and THC, but they are NOT the same enzyme.
My proposal:
THCAS sticks the landing when making D9, and we get “enviromental” conversion to D8.
CBDAS is not optimized for THC production, occasionally makes D9. if it doesn’t stick the landing, because the catalytic center isn’t optimized for that, why wpould you rule out landing on the lower energy D8 at a higher rate than THCAS does?
We agree that in a test tube, you can alter the D8/D9 ratio by changing the catalyst/solvent/reaction conditions.
THCAS and CBDAS are different catalysts…the GBG is in a different environment in those two catalytic pockets.
Given the D8/D9 ratio is different in plants that have one catalyst vs the other, arguing that it’s NOT the difference in catalyst seems pretty fucking bold.
Youre not going to make thc a different way from the same precursor…
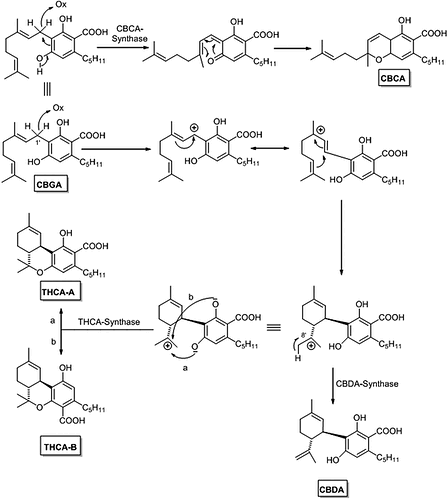
And the double bond always lands in the same position because of where the oxidation takes place… youre not going to make a “different feed stock” and be able to close that ring. Its pretty obvious when you look at the picture I posted above from that paper
No its not they both follow the same pathway till the VERY end…
Thats a bold claim to make when others have said the opposite.
Do you know what an enzyme is?
If the amino acid sequence at the catalytic site is different, then the environment in which catalysis is occurring IS different.
They take the same substrate, and their primary product is different. No way are the catalytic sites identical.
If you can’t see that, I see no point in continuing.
They are not idential, but very closely related since more then half of there function is the same. They literally both oxidize cbg in the same way the only difference is in the end.
Youre still not going to be able to close that ring and have the double bond shift to the 8th position! If you cant see that then i see no point in continuing either.
The reason it lands where it lands is because of where the enzyme attacks. Neither the thc or cbd enzyme will change where that oxidation occurs, and if it did you wouldnt be making cbd or thc or any kind
Yet that is exactly what happens with ptsa as the catalyst…
CBD=> D9 => D8
And you can change the rate of that last step. By changing conditions.
The conditions in the active site of CBDAS are clearly not optimized for D9.
You’re certain though that CBDAS won’t also catalyze D9 to D8?
I’m not certain it will.
But I am certain that such an hypothesis fits the D8/D9 ratios @Dr_Jebril sees better than your hypothesis does.
So youre admitting the enzyme isnt going to do it then. Because were not talking about acidic catalyst
If the catalyst can make either THCa or CBDa but makes them in a different ratio, it is a different catalyst.
It holds or guides the initial cation differently. That difference changes the CDAa/THCa ratio.
Only a moron would argue categorically that such differences could not alter the D8/D9 ratio as well.
Once again you prove yourself completely impervious to logic…
Good luck with that.
Not really
Cbda enzymes are cbda enzymes and thca enzymes are thca enzymes. Yes they can make different amounts of different things but that doesnt mean theyre not the same thing. Just like ptsa can make d8 or d9 in different ratios
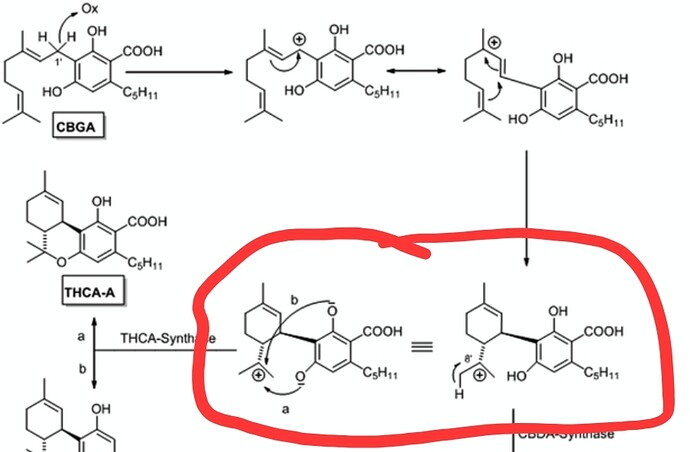
No it doesn’t… how dumb are you. The pathway is literally the same until the ring is formed… here i made this easy so even a retard like you can understand it
The molecule circled in red ARE THE SAME
The only difference is where which enzyme interacts. THEY BOTH WORK ON THE SAME PRECURSOR MOLECULE
Only a moron who literally doesn’t understand basic chemistry could think you could close that ring and make d8… its so easy to follow how this is formed idk how you cant follow it… if the oxidation took place in ANY OTHER SPOT the ring wouldn’t close… but hey I have to remember who im talking to here. Youre mad because youve been proven wrong and you want to start name calling
Say you lost the debate without saying you lost the debate
![]()
Yep.… exactly how they are held by the enzyme, and how much H2O/H3O+ fits in the pocket with them determines which way things go.
That is what is different between CBDAS and THCAS and how they produce different products from the very same precursor.
Nope. But the failure to find D8 THCA in CBDAS only lines would make me wrong. Not everyone has baseline separation of D8/D9, I’m only assuming that is true of D8/D9 THCA as well.
I don’t recall data either way. Do you haz?!?
Maybe @kcalabs has that data and can share.
Maybe Mechoulam has published it and I missed it.
I havent been able to find it either and ive been looking around for it all morning
Hhc is another one i cant find any information on, no one says anything about how its made other then it is in flower samples
I did find an interesting paper where they identified a bunch of new compounds in a cannabis flower sample idk why it wont let me upload
Idk why i cant upload this paper its only 300kb
Heres a link to it though from sci-hub.se
https://sci-hub.se/https://doi.org/10.1055/a-1110-1045
Its interesting because they didnt find any d8 in the sample but a bunch of other stuff
I wonder if they were looking for d8 though
Would the higher cis:trans d9 ratio in hemp be a possible culprit?
You wouldnt make regular d8 it would be cis/trans d8. The trans d8 would be regular d8. The hydrogens on the ring would be facing the opposite directions on the cis version
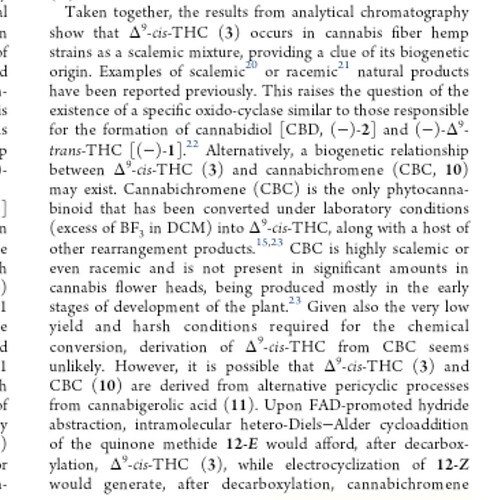
This is THC in the CB1 receptor I believe.
Hoped it was the synthase… nope. Really out to post refs with all the pictures.
Haven’t found cbga or THCa in THCAS yet.
According to a paper i found online they think the difference could be a oxido-cyclase that works on the cbg a scaffolding, i guess you can make cbc into cis d9 pretty easy
They found a direct correlation between cbc content and cis d9 content
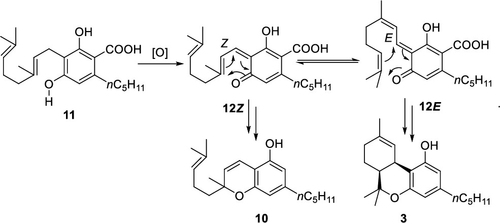
https://www.sciencedirect.com/science/article/abs/pii/S016816562300010X
Investigates directed changes to the active site of CBCAS to change the efficiency of the reaction.
Yet both THCAS and CBDAS can get you from CBGA to CBCA in vitro.
All about how the intermediate is coddled…
https://www.sciencedirect.com/science/article/abs/pii/S0168165618305698
We generated two CBDAS variants, C_S116A and C_A414V, with 2.8 and 3.3-fold increased catalytic activities for CBDA production. C_A414V additionally showed a broadened pH spectrum and a 19-fold increased catalytic activity for THCA production.
Single amino acid change at active site. Changes CBDA/THCA ratio.
Because the environment in which the reaction is taking place has changed…
If it was the case, we would logically see d8 in all d9 samples analyzed the same way, and in more significant amounts.
Heat alone do not seem to produce d9 from CBD, except in presence some of dirt (likely coal or tars, or some minerals or other catalysts). It does happen with dirty GC liners. One can see up to 0.4% d9 in 99% cbd isolate, but d8 remains below loq. This effect is proportionally lower in samples which contains 5-10 times less CBD.
The CBD:CBC:D9:D8 ratio I see in most (there are few exceptions) hemp is systematic lets say roughly 25:1:1:0.3. No matter the type/age (let’s say at least 2 years), cleanliness of the machine etc. We are dealing with low content anyway 0.05 to 0.25% max, most hemp being in the low to mid range.
In another similar thread on d8, we already discussed the fact that one should take the papers with a grain of salt, and not run run to the conclusions so fast. A lot of recent papers (and labs) are focused on d9 strains (no d8 or trace d8), or CBD strains below 5%, and often report LOQ above 0.05%. This review you are refering to several time does not prove, or even just affirm that d8 cannot be produced by the plant. They say it might be an isolation artefact (which is the case with many d9 distilates and reports seen on this forum), and further evocate that acidic isomerization can occur in plant material, not precising dead or not, but natural. It is a large review with many infos, but not a study really focused on d8 in natural CBD rich products.