@anon93688 @Photon_noir @krative @qga
What do you think will cbd ir cbd-a become more water soluble with an amino?
In University, I did a few reactions using azide click reactions and bromine activated pathways to make sugar drugs of a handful of NSAIDs. We used their hydroxyl and carboyxl functional handle in a way you’re describing. When it would not react, sometimes an amino acid bridge was required for steric reasons, like alanine, glycine and a few other alkane amino acids. I wonder if making a glucosamine-cbd or glucosamine-cbda would be a fun route (or even galactosamine). Pair sugar with activated hydroxyl or carboxyl group and wham bam you’ve got something that (hopefully) your body will metabolize differently or quicker because it would recognize part of the structure as a native molecule. Or the amide bond wouldn’t break as there isn’t an enzyme to my knowledge that would process the bond in your body.
I’ve already asked if OP wanted an amide coupling SOP.
Is it safe?
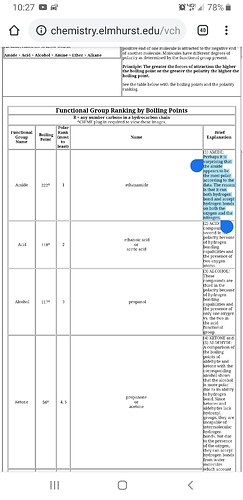
Cannabinoid amides have been used in formulation for some time now.
RIP to those who use thionyl chloride and don’t take proper precautions.
This is also teaching people to dump and stir chemicals without ever purifying them.
Noted, after seeing the cbn thread I figured there was enough common sense here to look up some lit. Without spelling out stuff.
@ScoobyDoobie, it depends on the size and functionality of the rest of the amine group. Amino acids individually are sufficiently soluble in water, but adding one to a cannabinoid is not straitfoward (it would be a covalent bond of some sort, not ionic, as you may be thinking), it is unlikely to make the cannabinoid water soluble enough (though it may increase it slightly), and an amino acid group will certainly change the cannabinoid’s activity!
An amide might be easier to add to a cannabinoid acid, and it may enhance water solubility (especially if you let the N+ stay hanging and retain the cannabinoid’s carboxylic acid, making an internal salt or zwitterion), but it will still change its activity.
You’re now getting into the territory of synthesis of new compounds rather than just doing temporary modifications for solubility or isomers… so @Krative 's question is quite valid: Is it safe? …in creepy Running Man dentist voice…
It really depends on where and how the moiety is attached to the cannabinoid. Bee careful, folks! ![]()
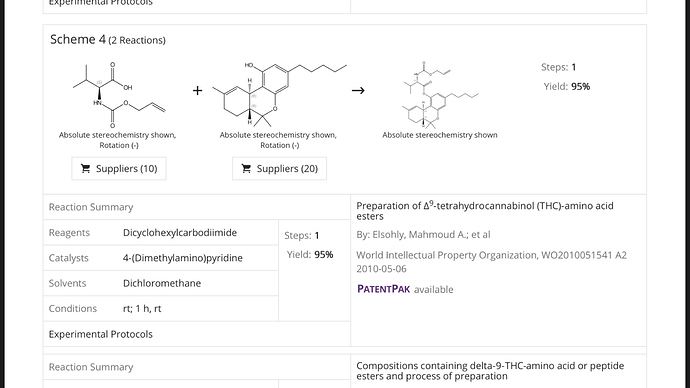
These amide couplings are reported processes.
You can use the hydroxyl group from phenol or you can use the acidic cannabinoids for the coupling reaction.
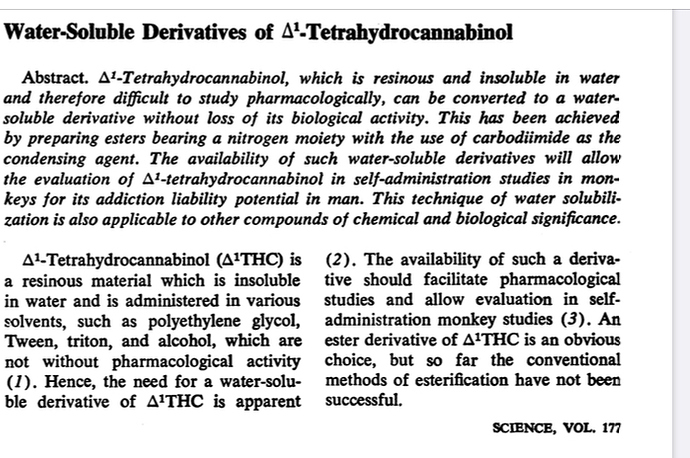
Here is a publication on this subject. The water soluble THC-amides are hydrolyzed in your liver back to THC and get you baked.
The only catch is that an amide coupling reaction is not atom efficient and will not scale so well
As in reported formulations for research or products currently being sold?
There’s an amide coupling reaction that was used widely for production of LSD if I’m not mistaken. Cost effectiveness is Different with a substance with microgram potency
Not true, look into the reagent PyBOP
Would your liver be able to metabolize the water soluble THC-amide faster than say sonicated nanoemulsions?
Sonicated nanoemulsions are technically not water soluble and more so a drug delivery method and are metabolized just as THC would be.
THC-amides are completely different molecules and act as pro-drugs to THC, if you smoked it it wouldn’t get you high. Its similar to how vyvance is dextroamphetamine with a lysine bound and only converted to dextroamphetamine via liver enzymes.
Nice, here is the file if anyone wants to download.
zitko1972 water solunble thc.pdf (692.2 KB)