Based on the limited info I have (because I didn’t go back and read through this entire thread) LC/MS is what I would suggest for as close to “absolute certainty” as possible.
You have to remember that LC is the separation and the MS is the detector. So if you don’t get great separation, then your detector can struggle with providing certainty (which is a factor of repeatability/reproducibility/accuracy/precision, all of which are/should be covered in the method validation, provided you have a good chemist).
That being said, mass spec uses a different type of detection method (as opposed to UV/VWD/DAD) called a “mass to charge ratio” that’s essentially unique to each analyte under investigation. This is why mass spec is used in clinical toxicology (how i was trained), especially as it pertains to drug discovery. In other words, you have to have quite a bit of certainty when analyzing a rehab patient’s urine before you report a positive result and then potentially ruin that individuals life. So I’d say it’s as close as you might be able to get.
When it comes to the data analysis, it’s possible to still have false positives for a number of reasons (usually chromatography related), but some data analysis programs give you an option to download a chemical “library” which acts as a reference database, allowing you to actually look at the statistics of how likely the peak is to be a certain analyte.
So “certainty” I think would be a factor of the lab you’re using, the skill of the chemist, the equipment, and the sample itself.
Also, I’m honored that you thought of me on this thread ![]() @cyclopath
@cyclopath
Thanks for taking the time to break that down. That’s one of the better explanations I’ve gotten as to how and why on testing.
If you aren’t an expert on MS interpretation (which is hard), a GCMS is actually more helpful for unknown characterization. The reason is that the common spectrum libraries have like 100x the compounds for GC than they do for LC. Often times a library scan on LCMS turns up nothing useful.
Yeah I agree with you on this one. All the libraries I’ve seen for GCMS are WAY more extensive than LCMS.
LCMS has unrivalled ability to detect things that you have reference standards for. Unfortunately answering a question like “what’s the missing 10%” can be an arduous process requiring multiple instruments and extensive analysis. And there is no solution that works for every possible compound.
A GCMS is the best first place to start. If your compound either isn’t in the GCMS library, or is too fragile or heavy for GC to get to the detector, then you’re in for a long haul. Preparative chromatography leading to NMR is a commonly useful approach. Elemental analysis, LCMS, and physical properties are also commonly helpful in narrowing down possibilities.
for some reason I had assumed the reverse would be true. that getting things to the detector was easier to accomplish via LC than GC. because some things just don’t like being heated…
If you dont want side reactions start with isolate
I can get just about a full conversion of cbd to d9/d8 using a certain acid and solvent in a reflux
Side reactions only happen from impurities (degradation can happen too but that’s different from a side reaction, usually you’ll get cbn when you degrade thc)
Gcs were used for analytical testing/urine analysis long before HPLC was even around which is why they have a bigger library from my understanding
I’m going to re-interpret that as
Mass specs are easier to hook up to the outlet of a GC that an LC (MS requires vacuum)…so GC’s were hooked to Mass Specs earlier.
because LC came waaaaay before GC
edit: although it looks like the GC beat HPLC to the table…
Chromatography dates to 1903 in the work of the Russian scientist, Mikhail Semenovich Tswett,[4] who separated plant pigments via liquid column chromatography. German physical chemist Erika Cremer in 1947 together with Austrian graduate student Fritz Prior developed the theoretical foundations of GC and built the first liquid-gas chromatograph, but her work was deemed irrelevant and was ignored for a long time.[5] Archer John Porter Martin, who was awarded the Nobel Prize for his work in developing liquid–liquid (1941) and paper (1944) chromatography, is therefore credited for the foundation of gas chromatography. The popularity of gas chromatography quickly rose after the development of the flame ionization detector.[6]
Following on the seminal work of Martin and Synge in 1941, it was predicted by Cal Giddings, Josef Huber, and others in the 1960s that LC could be operated in the high-efficiency mode by reducing the packing-particle diameter substantially below the typical LC (and GC) level of 150 μm and using pressure to increase the mobile phase velocity.[3] These predictions underwent extensive experimentation and refinement throughout the 60s into the 70s.
The reason is because LCMS is much newer than GCMS, so there hasn’t been as much time (or investment) in filling out the libraries. And also if you’re using it in “scan” mode, you get none of the benefits of tandem MS/MS. So most people who buy LC-MS/MS don’t go into it with the intention of using it that way and it’s not a big selling point.
Gcms was used for urine analysis up until a white paper in the 90s was written about LCMS and that’s when the industry changed (@anon6488101 is the one who told me this a while ago, she ran her dads drug testing labs in FL)
Right, and in routine drug testing, they have little need of unknown characterization because they’re usually just matching a list of watched compounds for which reference standards are readily available. Tandem mass spec is perfect for that.
I’ve discussed the generation and utility of those libraries with a PhD Chemist who actually taught himself to program just so he didn’t have to do those “library” matches by hand (eye). kids these days…
Yeah I never actually met another analytical chemist from toxicology that knew how to use the scanning feature. There’s a lot of opportunity for r&d with mass spec if you know what you’re doing. I guess my only concern with gcms in this specific scenario would be how the analytes hold up under high temp, or the kind of separation that’s needed for accurate analysis - like @cyclopath mentioned. If gas chromatography isn’t the best method of separation for the cannabinoids in question, then I suppose it could be used for qualitative purposes instead? Perhaps as a “research” step (using the extensive library) before developing a proper method using lcms?
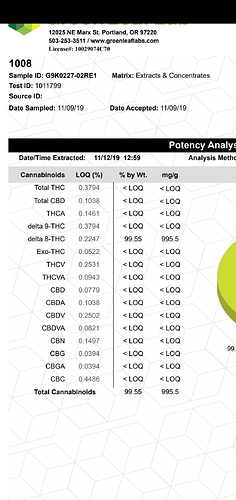
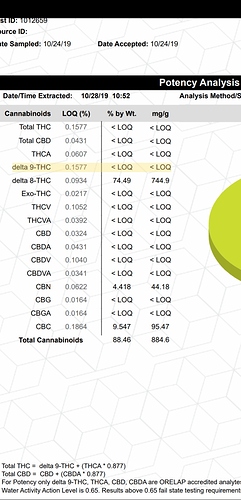
One of the reasons we don’t use gc for thc/THCa identification (among others) is because the analyte decarbs in the method, so we can only see “total” thc which Im sure you already know. In this case, I guess I would look into the stable/unstable temperature conditions of the analytes we’re looking into to know if gc is even an option. But I may need to educate myself some more on the nature of those compounds to have an opinion
Therein lies the rub – if your question is “what’s in the unknown 10%”, you don’t even know that much.
What kind of d9/d8 percentages are you seeing with that method? Are you able to control the percentages of d9/d8 based on residency time, amount of acid and/or solvent? Temperature? I saw a chart in another thread that seemed to show conversion based on residency time, but can’t remember exactly what it was in relationship to (d8/d9 or cbn) Tell us more.
The method I use makes 90-95% d8 with 3 to 5% d9, I’m using this to make cbn so its perfect for me
I’m working on tuning it in to produce no d9, I think a higher boiling point solvent might help with that since you can isomerize d9 into d8
HCL can produce pure d8 if used correctly (this is not what I’m using, this is my buddy’s method)
Damn, that’s the best lab I’ve seen on a conversion so far.
Any idea how citric acid or t41 in the boiling flask perform in comparison?