Just curious why do you prefer ethanol/methanol with the p-toluenesulfonic acid?
The patent I’ve seen for d8 uses toluene and p-toluenesulfonic acid.
Have you tried using heptane and refluxing in your 100l reactor under nitrogen/argon with that method yet?
I will say that’s impressive only having to reflux for 47min @ 63c to get a full 1:1 conversion with 13kg of isolate.
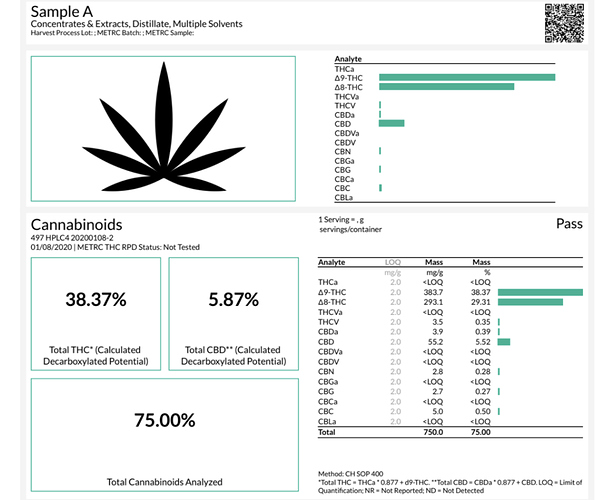
Since you shared your 1:1 sop I might as well share mine and a coa of what I got using cbd distillate:
Cbd to 1:1 D8:D9
Materials needed:
Heptane
T-41
Silica 60a
T-5
Celite 545
Short path distillation setup
10l heating mantle
10l 3 neck boiling flask
Vacuum take off adapter
Reflux condenser
Rotovap
Ballon (large punching ballon works well)
Argon or nitrogen gas (I prefer argon)
Process:
Heat 1kg cbd distillate to 100c
Add 2l heptane
Mix well and then pour into 10l boiling flask (add stir bar to flask first)
Set stir bar to 400 rpm.
Slowly add 320g T-41 (32%)
Add reflux column, thermometer adapter and valves to reaction flask. (Have chiller connected to reflux column and set at as low as possible)
Pull vacuum on flask and then back fill with nitrogen to inflate ballon (repeat this 3 times)
Heat to 90c for 4hrs
Turn off mantle and wait for mix to cool
Filter out T-41 over celite 545
Filter solution over a crc style column packed with 125g t5 bottom, 250g silica 60a & 125g t5 on top to neutralize ph (25% of each powder)
Rotovap out heptane
Distill oil as normal on SPD
I should add this sop only works well with cbd distillate and when I tried it with cbd isolate I got mainly d8, thinking it needs to be ran at lower temp/less time.
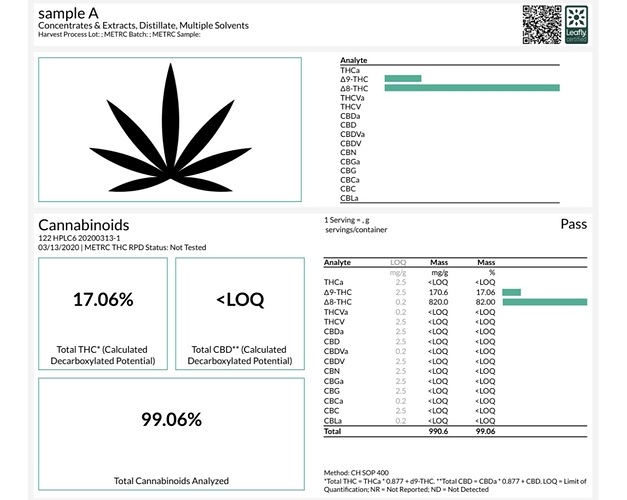
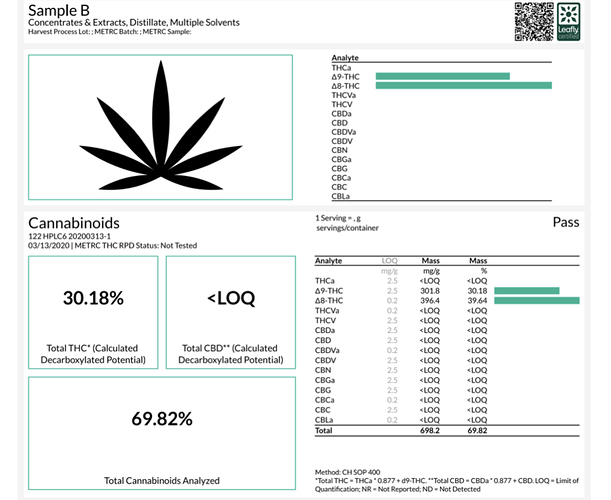
Here’s what I tried and results with isolate (I do think the results on sample A were off since I’ve never hitten more than 90% TAC with my spd.
Sample A: 1kg isolate heated @ 90c for 4hrs
Sample B: 1kg isolate heated @ 75c for 4hrs (accidentally overshot temp to over 80c for first hour then 75c for remaining 3hrs)