Industry and educated Chemist with background in multiple SOPs developed personally and proven to convert CBD to 70% D9/20% D8 and up to 80% D8 in other preferred methods using no DEA listed items. Looking to perform these for you personally. DM for further questioning.
Serious you are a chemist and only get 80% D8
70/20 D9
Time to hit the drawing board
Edit : could have comented less harsh
My appolegies for that kids and wife 24/7 is a whole new experience ![]()
![]()
What sort of industry and what sort of education?
What’s the other 10%? Lol
CBD left over from starting isolate
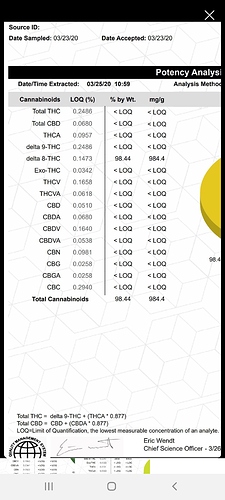
To your point @Roguelab 80% D8 from starting is not the goal but through minor and large batch runs, 80% D8 using not AC but other Leiws based acids and maximizing yields and run times is fantastic with under 20% loss from starting material, a batch of converted isolate starting at 96 - 98% isolate to a 70% D9 and 20% D8 is a fantastic product in the scale of reaction for synthetic chemistry. I understand the shade however feel it is misunderstood. Not everyone can afford to convert under a pressurized vessel to achieve 90% D9, let alone run a prepatory chromatography setup to clean up residual isomers. Thank you for the educated dig as a respected contributor. I too am always questioning the validity of others.
The special sauce
Sounds like your running an untuned metal salt conversion. What’s the TAC of your reactionary crude and what’s the TAC of your distilled material?
No salts are used in this conversion if referring to the well known German patent. So no TAC necessary here. Great question. I have not tried the ZnBr2/ZnCl2 reaction but I do not recall, does the patent state a limiting factor of carbides in their synthesis?
TAC is referring to total active cannabinoids…
Do you have in house analytics for validation?
You should be able to get a full conversion to d8 if you use isolate if done correctly
Shoutout to the homie who gave me this SOP
In your question it may have been, I have never heard of TAC referred to as total active cannbinoids and yes I do, I run two 1100’s and a 7000D GC/MS. TAC as you’re referring to of input is 96-98 % CBD isolate, after conversion, a rolled film is used to remove any bound solvent. No distillation in performed and measurable TAC (again your acronym) is at 70% D9, 19.8% D8, with the remaining being isomers moving through the chromatograph, currently I am working on using an NMR to identify what those isomers may be.
My goal has not been to produced D8, rather a high D9 and the D8 number came from the improper preparation of the constituents and environment from which it was ran under, I would love to move on from this synthetic conversion to D8 solely, however it was not the original goal and thus I cannot speak to its conversion metrics as of yet, those are great numbers coming from an isolate. I imagine this was done with one of the many patented methods available or a reflux in a short path using bleaching clay’s for a number of hours?
Idk TAC is pretty widely used; it’s an important metric in conversions. If the conversion is clean. The TAC should be more or less in line with the starting material.
Really sounds like you have to get yourself a better isolate plug. 96% is pretty damn low that stuff would be yellow as fuck; poor starting material is an excellent way to invite unforeseen radical formation.
Maybe TAC is widely used in California, as through my experience I have not ever heard of this to date, but will make sure I make note of it moving forward. Thank you for the input and added knowledge.
In response to the color, this does effect purity, only in the slightest do to residual oxidizing agents, but also speaks to the number of washes performed and the solvent that may have been used to do so, pentane under proper cold may wash color out with as little as one wash, heptane, being a larger HC organic may take up to five, the isolate numbers are also dependent on the method of winterization. I am enjoying all the feedback, its nice to always being utilizing a community of minds for continued growth.
No bleaching clay and no reflux
If you’re looking to make high purity d9 you need a weak acid and an inert atmosphere
Such as say an argon and concentrated Lewis that yields a weak nucleophile?, say H3PO4? Or H2SO4? Both leave fantastic results but far to spead electron charges in their nuclephiles, thus why I think the metalliic salts were such a big hit early on due to their concentrated nuclephilic charges. I have moved to another Lewis acid and thats how I got the current results originally posted. Very cool to see the knowledge and understanding here @Kingofthekush420, hope you are still playing with these safely.
@Kingofthekush420 what do you consider a weak acid? im refluxing ethanol with p-tsa and getting 55% d9 and 25% d8. looking for higher d9 potency. I have a glass lined steel reactor in a C1D1 environment so i Have the ability to run pressurized, and have pentane, hexane and toluene on hand, along with HCL acid, citric acid and P-TSA. i dont have any sops on pressurized conversions as of yet though. @Roguelab could you give some guidance? btw all the open source tips are crazy awesome. we will all benefit from open source sharing in the end, as long as its reciprocated. another question… why cant we do acid conversions in stainless vessels? what is the dangerous by-product that comes from 304SS and acid? that is the only thing stopping me from scaling up is finding a glass lined reactor a larger size.
Just my 2 cents: Substitute the ethanol for an alkane like n-heptane.
Are you pre-drying your CBD solution with activated zeolite or sparging it prior to use?