Im trying to find data for atmospheric BP of cannabinoids and finding only .5mmhg reports- internet is swamped by the typical distillation temp under vac but what damned temp do they vaporize at atmosphere?
Sigma has a nice little nomograph application that could help you estimate it. You need two points to get atmospheric, but it shouldn’t be too hard to do.
I’ve heard the boiling points are on the order of 400C. At that point boiling seems moot, even in an inert atmosphere since we are getting into pyrolysis territory!
Moreso looking to determine relation to some other compounds in the mix
Attempting this without some kind of protective atmosphere would surely lead to extensive degradation/fragmentation/rearrangement.
The rule of thumb states that all bets are off once you heat a molecule to 250C or above. There are of course exceptions but I doubt the cannabinoids are among them.
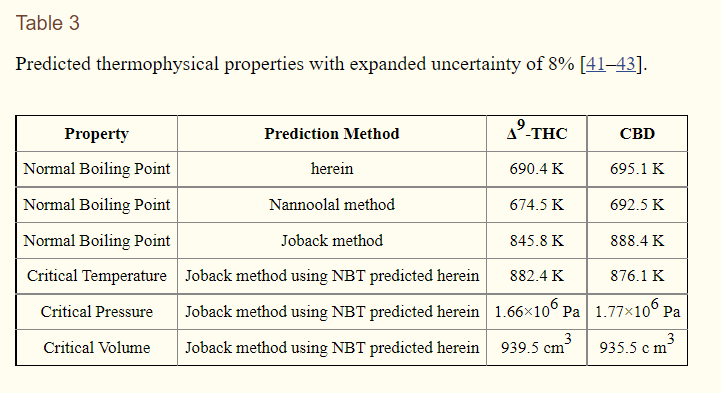
That said, the extrapolated boiling point has been carefully arrived at in a study by NIST in their efforts to develop parameters for a THC breathalyzer. I’m pretty sure the authors used the data they obtained in conjunction with the Clausius-Clapeyron equation and arrived at an extrapolated standard pressure boiling point of 418-418 degrees Celsius.
Wouldn’t point of vaporization be pretty close?
I’d think THC is in the ballpark of 400°F, probably a little less, maybe a little more.
An enail does just this, and the goal in my mind utilizing it is just a hair above boiling point.
F or c
Yup I usually run an enail at 420F
Here’s what I found when looking up your question.
It covers vapor pressure at lots of temperatures and pressures. Its specific about CBD and THC - but not the others. And it shows the different methods you could use potentially to extrapolate this information and how you can roll an experiment to collect the information yourself.
They did the experiment and showed that the difference between reality with controls and the prediction is pretty darn close as well.
So the boiling point of THC is 420C? Nice. Thanks god, err physics. Whoever did that one.
Yes, that’s the NIST study I was referring to.
Yep this is the paper I use to help determine my still body temps based on vac pressure
Oxygen causes acids and other compounds including cannabinoids to ignite with full atmosphere at the temps you are looking for. I think what you are looking for is what is the temp where cannabis will create a vapor stream under a minescule in flow motion of atmosphere.
On a side note if you pressurized a vessel with specific inert gas and crank the temp up it will not affect it at all. And can handle scorching temps.