Thank all of you for your help
Hello everybody
This is the lab that produced above chromatogram. The sample was indeed very dirty which resulted in the noisy baseline. We did a proper peak assignement and there was no d9 present. The signal at 9.956 min was overlapping with d9 but had clearly a different signal onset when comparing with known samples. The same applies to CBD with the signal at 8.065 min which again overlaped with CBD but had a different onset.
There was no CBC (usually right before CBD) and no CBG (usually between d9 and CBN) despite the numerous signals everywhere.
We are confident that another lab with a comparable separation performance will give similar results.
I hope this helps you further. You can always call the lab for more information.
Best regards
Dr. M. Guttentag
CBD-Test
We see the d9 similar to your 9.956 peak.
There should be a couple other isomers in there.
Signal onset is used for marking integration start. It should never be used for peak identification. I would like to hear your explanation on how exactly the signal onset from the sample is CLEARLY different when compared to a standard. Do you have chromatograms to help visualize this statement?
Onset was used as a secondary identifier for confirmation. Primary identifier was the retention time which was already off.
Retention time shifts between clean standard and dirty matrix are fairly common. With how dirty this matrix appears I would almost guarantee some slight shift in retention time.
Just curious, what solvent are you using for standard prep and sample prep?
That peaks looks too large to quantify. Why wouldn’t they dilute the sample during sample prep?
Can in fact be possible.
I’m using a similar column. It makes very nice separation with sufficient time and convenient program.
Here is a similar type of sample:
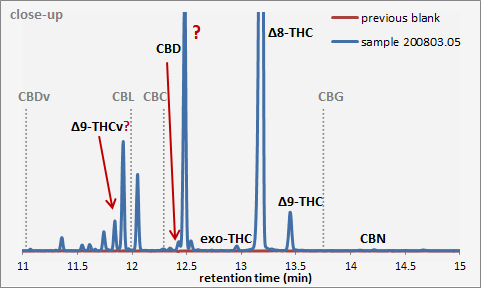
Out of the 10 I can test for, 4 are not showing up for sure.
The only confusing one is THCv. I believe here it is not him.
Also dealing with CBC, there could be something, bud way too small, and there is not reason it would actually be there. So here it is discounted.
Tbh that sample still looks a lot cleaner than this one.
I think so too. ![]()
Still here it “column compensated” (previous blank was subtracted), and conveniently zoomed on. A bt polished regarding the usual output of the chemstation.
Would you have any tips on separating d8 and d9 on a GC-FID? Any column recommendations?
I generally just yell at the D8, and treat it like the red headed step child that it is. While simultaneously whispering sweet nothings to the D9, and try to coax it out on a date with my endo-cannabinoid system… Hey, if it works, it works!
But seriously, KCA is who I’d ask, so you’re on the right path…
Hope so ![]()
I bought an ancient HP 5890 off ebay, haven’t gotten it into place to test it just yet. I’m gonna try with some second hand columns just to see how it turns out. May end up with the Restek columns but you never know, maybe theres better options ![]()
A phase 5 column works well for D8 and D9,but you can get good separation of all cannabinoids on a phase 35 column. CBD and CBC will coelute on a phase 5 column unless you have an extremely long run time. Same for CBG and CBN.
Exactly what @bigbone said wrote above. ![]()
So far I have used DB35-ms with success.
Now Im trying it for derivatization method, bur Im facing quite some difficulties with d9 and an unknown compound from reageant (only with non decarbed mixes). ![]()
Thank you both for your replies. That’s very interesting. I just so happened to pick up two used columns on a whim: DB-5MS and DB-1701 60 m X 0.25 mm X 0.25 um
I’m guessing the DB-5MS is a phase 5 column. I’m guessing the other is a phase 17?
Should these be an okay starting point at the very least (my machine has dual FID if that factors at all)?
Does this refer to the process of using MSTFA for acid analysis?
The main phase columns (1, 5, 17, 35) are all methylpolysiloxane modified with a certain amount of phenyl groups (the number gives the amount of modification). The 1701 is a little different, being a cyanopropylphenyl modified methylpolysiloxane. I don’t have any experience with it, but I am pretty sure it is medium polarity so it might behave similar to the phase 35 columns. Would be interested in the results.
Here is a good link for cross-reference of columns between vendors: GC Column Cross-Reference
If you want to know more detail about the column look up the USP nomenclature in the third column.
Thats very useful, thank you!
Question about column length. Does the longer the column improve the separation (assuming at the expense at operation time)?
Exactly. Better separation but longer run time.