Hi, I’m looking to run my recirc chiller (polyscience ADR unit) down to the -40c but don’t have access to any special heat transfer oils where I’m at. Wanted to run this weekend rather than wait a week for a amazon delivery
Can U just fill the reservoir with 200 proof ethanol? I’m talking 15 liters in a well ventilated area on a patio for about 3 hours operation only.
Thoughts?
Figure that’s far better than potentially ruining seals with an antifreeze mix from walmart…
You’re more likely to ruin seals with a petroleum based fluid if the seals and interconnecting hoses aren’t rated for it
I’ve run denatured ethanol in a Fluid Chillers unit that was rated for use with flammable petroleum based fluids (Dynalene HF-LO). As long as your pump, lines and seals are rated for it you shouldn’t have a problem doing so. Fluid Chillers required the dynalene to maintain warranty but once we were out of warranty and had a leak the denatured ethanol was a whole lot cheaper per gallon to replace it with
I don’t know the ADR specifically, but most of the Polyscience chillers are rated for use with a glycol based coolant, at -40 it just needs to be a very strong glycol to water ratio to keep from turning into slush or freezing
4 Likes
The Polyscience info online is adamant NOT to you use anti-freeze (glycol) in the reservoir.
I’m using closed loop to chill down a small jacketed steel vessel. have silicone hoses rated to -60C.
have 5 gallons of heptane denatured EtOH.
Sounds like I can just fill with 3 gallons of the denatured EtOH I have, keep the cover on the reservoir and I’m good to go. Obviously I’ll probably lose a few % points of my EtOH to evaporation before this test is done… but I can live with that.
my unit is a polyscience ad15r-40-a11b
Appreciate the practical experience comments!
only if the manufacture lists it as an acceptable heat transfer fluid. there can be different parameter’s that you need to run at when using ethanol in a chiller
we use pure 99 etho in out hubber, the only additive i have used for other chillers is like 10% methanol or methalhydrate to insure there is no moisture in the chiller
Never use ethanol in a chiller unless the chiller is rated XP and C1D2……
Not only that, you’re pumping flammable solvent in and out of the control area using non-rated equipment.
This is a big no no…
5 Likes
agreed. unless the chiller is designed to run ethanol. just say no
1 Like
Understood and respected.
1 Like
It wont use the full range of ur chiller, but u can always use 30% alcohol/70% water mix to get something that goes below 0c without freezing up or catching on fire.
30% ethanol mix should get u down to -10C without freezing over
1 Like
When a glycol chiller is running r290 as it’s refrigerant, does that mean you’re able to go down to -60c on your glycol and/or recirculating chiller fluid without trouble?
There’s charts on the back of automotive coolant jugs showing freeze point vs concentration. As with everything, it’s a trade off. More water means higher freeze point but more thermal capacity per unit of mass or volume. More glycol means lower freeze point but less thermal capacity.
That said, within reason you can replace capacity with flow which is why at relatively low temps it’s a lot easier to pump denatured ethanol, ethanol, methanol or dynalene hf-lo than a slurry of glycol and water
2 Likes
Just to add, viscosity also becomes a consideration as you drop temp. Your pump will have to work harder to push sludge and could wear parts early.
German chiller companies rate their chillers against ethanol, but youll see the DIN rating against ethanol stops at 10C, because the flash point is like 13C. After that the real world performance is rated with thermal oils or water.
1 Like
Absolutely not. But you might manage -60F, just…
Pure water freezes at 32° F, but a 60% solution of ethylene glycol pushes the freeze point down to -60° F.
Using Gylcol In Closed Loop Systems | A Complete Guide.
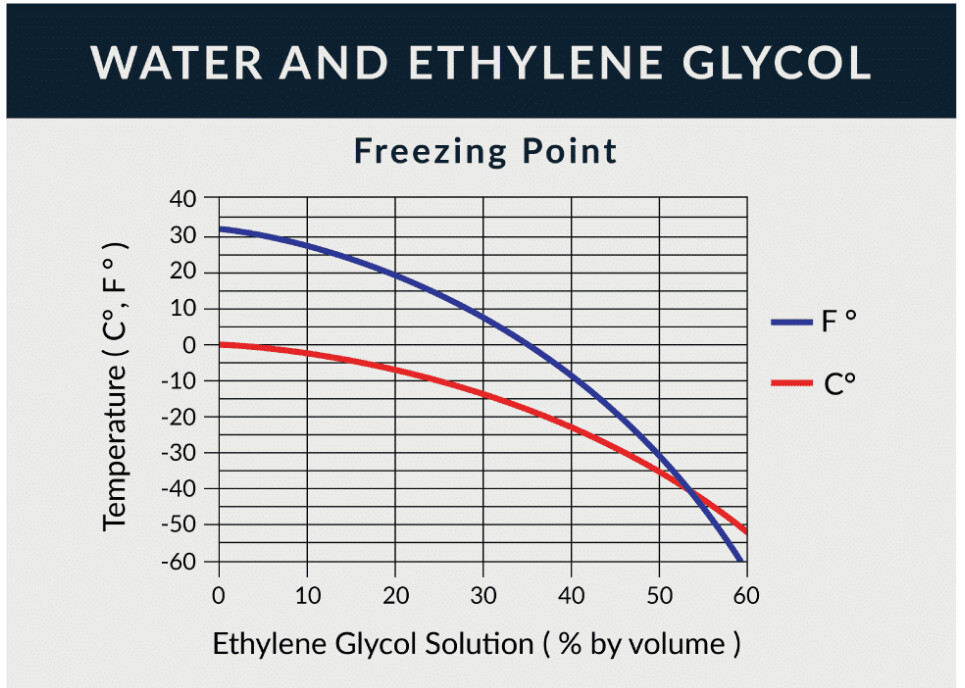
The above are “freezing” points, and as both @greenbuggy & @anon45638961 mention, you get hard to pump slush first.
@PCGextraction
Presumably that’s Methyl-hydrate?
Which is simply another name for methanol (for those who have not shopped at Canadian Tire).
https://www.canadiantire.ca/en/pdp/solvable-methyl-hydrate-0497127p.0497127.html
haha yea that’s what i meant for sure. Are you from Canada?
Like @lightbulb? Nup. Cannada 
… although I have used Canadian tire to solve canna related problems.
2 Likes
Use 200 proof iso, it’s a bit more stable when cold. Don’t use water as the chiller will rip the water off the fluids and coat the heat exchanger and possibly cause a failure or damage. The caviar is you can’t use also hold at about slightly higher than room temp unless you are trying to welcome a accident. When using water with oh or similar the temp doesn’t exactly freeze till you get to a lower temp but the fluids are so dense that it almost always damages pumps. So it’s balance act. There are some diathermy chemicals that are altered water(don’t remember the compounds) and these liquids don’t allow the water to completely separate and coat the insides with ice. Iso is good, but only at low low temps. And it must be sealed so water or air moisture stays out.
again DO NOT DO THIS unless your chiller is rated for alcohol!
1 Like
