Hello everyone! This is my first post here. Recently I tried to oxidize CBG and got a peak which was around CBC on my hplc. I sent it out for testing and this is what I got. Anyone got an idea what that unknow peak might be? I’m planning to send it to the chemistry department at the university here to identify it with NMR. But it will take time. I was wondering if anyone here can give me a lead of what I’m looking at. Thanks!!
How did you oxidise it ?
Oxidiation of CBG normally goes along this chain of reactions:
CBG → CBC → CBTC → CBL + iso-THC
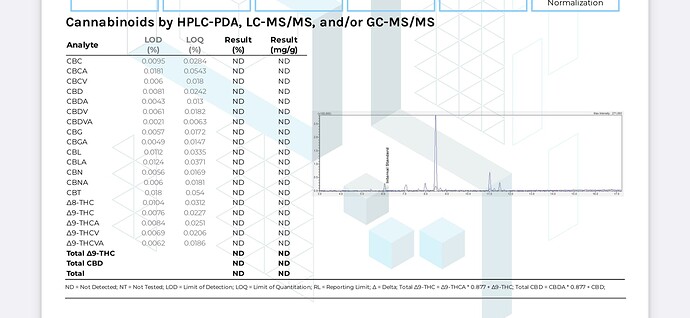
Here according to the COA, there is not any CBG/CBC/CBTC/CBL, so that is strange. ![]()
NB: the nomenclature of a pair of classical cannabinoids have been recently updated. CBTC refers to cannabicitran, and CBT now refers to cannabitriol. I believe that @kcalabs here reported cannabicitran as CBT.
Yes, we report cannabicitran as CBT. Looks like we should change that.
Did you rule out some possible presence of iso-THC ? (eg by comparing with a semi-synthetic d8 sample)
We know where the iso compounds elute for the various homologs, but I’m not sure they looked into this with that in mind considering we didn’t see anything we could identify.
Did the lab share the MS/MS ions with you for the peak? Is the university doing NMR / IR as well? I mean…someone else has already run GCMS (according to the COA screenie?)
Actually the exact method is not specified on these reports. Because the acids are listed, I guess this is HPLC-DAD data.
Agreed with @Cassin, need to have NMR done as a secondary technique to actually identify the compound if it is truly an unknown. Doing solely mass spec is only partially acceptable if we’re talking about the actual identification of a peak.
That peak needs to be isolated through prep chromatography and then analyzed through at least two techniques that can compliment each other for actual identification.
Unless we are confident that the peak in question has a very good chance to already be something assumed and there is a standard solution out there to compare it to, you’re going to just get a random library match using solely mass spec for identification, and that isn’t really acceptable for good science.
These data are a mix of assays, both HPLC and GCMS.
I just reacted it with acid. Don’t remember the exact parameters I used. I’ve to check my notebook. I did several experiments. All of them showed that peak and identified it as cbc on my hplc.
Yes sorry I meant NMR. They helped me identify CBN acetate when the labs were not testing for it.
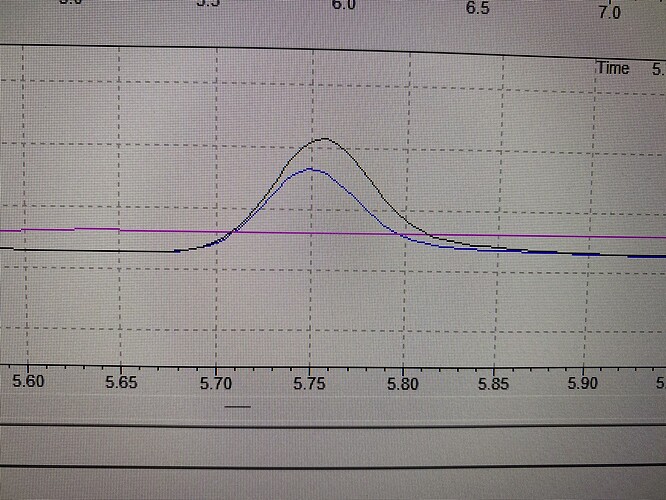
I compared my d8 data with data from these experiments. There are no matching peaks among them. Here black and blue lines are from 2 batches of this experiment and the pink line is d8.
Ha! If you used an acid then it is likely not undergoing an oxidation reaction such as I pictured in my first reply.
It is rather an acidic isomerisation forming “abnormal” CBG (abn-CBG). In fact there should be two species (which I believe are two different abn-CBGs).
@kcalabs Do you see only one peak with the two different methods ? No secondary species ?
EDIT: in fact I’m mixing up different reactions. My comment here is wrong about abn-CBG ![]()
I’d have to go back and look. I’m assuming I know what sample we’re talking about without asking who @Scorpio is, but it might be best to get a sample ID sent over to me.
@Scorpio, if I’m correct, is this the same sample we looked at twice a couple years apart?
What is that? I didn’t even know about abnormal CBG. How is it structurally different from CBG? Are the health benefits same as CBG?
It’s a sample from early August. I dm’ed you the sample id.
Am I tripping that there’s only one abn-CBG? The two -OHs and -R group are in the 1,3,5 positions - you could swap the -R with either -OH to make equivalent molecules (i.e. abn-CBG).
Do you mean that this second abn-CBG would have a substituent at 2 or 4 position?
I have experienced an unknown peak with cbg oxidation routes as well. Was under the impression it might be exo-thc aka d11thc.
I have a 15 standard library and some others that were marked without a standard (thco / hhc) and nothing was close.
Makes a runny product that’s similar to cbc but definitely is not cbc.
https://www.instagram.com/p/CRfN4a0ANFbXc54_K79bT0qHyvKC9000CxmPu80/?igshid=NDRkN2NkYzU=
Good information. I think we’re leaning toward acid catalyzed isomerization as the route rather than oxidation. Any experience with that?
For the unknown product I had made I was trying out the chloranil oxidation process. Though I do believe chloranil is slightly acidic to begin with and would concur with your statement and may be a contributing factor.
During the reaction, you get chloranilic acid as the byproduct as hydrogen is scavenged from the CBG. There is a paper on its use for making CBC and figured it was worth a try. Definitely made something but it is absolutely not CBC.
CBC, CBD, and the THC’s are all isomers of one another (C21H30O2).
Traditionally it was thought that exo-THC (d11thc) was only made through pure synthetic reactions, but it might be possible to also make it from CBG or other cannabinoid, since after all, it too is just an isomer of the C21H30O2 group.
Personally, I am only leaning toward exo-THC as its one of the few remaining standards I need to add to my library. It is definitely not any of the following: CBDV, THCV(8/9), CBT, CBL, CBND, CBC, CBD, D106a, D10, D8, D9, CBE, CBG, CBN, CBF, CBX, THCo, HHC, or Olivetol. ![]()