I agree with @emdub27, you would need a pH closer to 7 to increase your S more, which means you need quite aggressive chelates or micronutrient concentrations to avoid Fe issues. There are other ways to get S containing molecules into plants though (which are not sulfate), which can better achieve the goal of higher S in flowers and “skunkier” smell if this is your preference, sadly NDAs forbid me from sharing more on this front.
Have you ever used thiosulfate in a soilless medium? It’s on my experiment list.
Hey Daniel. Yes, I agree to know what it is would require testing. But my goal was simply to guess what it is based on the available information while assuming all their claims are true (for example, they’re using a chelate). And I do think it’s a liquid because the use rate is 1-2 mL per 5 L. I doubt they would give a use rate in such low volume if it were a solid. Given those considerations, I think calcium citrate is still a reasonable guess.
Thanks for your comments.
If the use rate is quoted in mL then it most likely is a liquid. While citrate is a potential guess, I dont know if it would be a reasonable choice - if you want the Ca to be chelated - given that a lot of HCl is needed and that in strongly acidic pH basically all the Ca is unchelated (citric acid is just present in its completely protonated form and does not chelate Ca).
Another possibility would be Ca acetate + several potential amino acids that form chelates with Ca. This mix can be acidic, chelated and contain no chloride.
I agree that it could be other chelates and mentioned that in my first post.
The reason I’m guessing calcium citrate is that it requires a dilute aqueous HCl solution to dissolve above its water solubility limit of 0.95 g/L at 25’C. And the marketing claims the product can be used as a pH down solution, replacing phosphoric acid and nitric acid (sulfuric acid isn’t commonly used as pH down in commercial products in the USA). So, a dilute HCl solution would seem logical guess because it acts as pH down, and they tested for most other elements except for Cl - because I assume they don’t want to scare growers with a high concentration of Cl, or to protect their IP.
I think their use of “nano tech” and “chelate” is for marketing more than efficacy. So if my guess is correct, while it’s true they could be using “nano tech” (with a loose definition) through micronization and chelates, the final product is neither of those. So the Ca would be in solution, not “nano” or chelated, and the chelate would be protonated.
And if I’m correct, considering ca citrate solubility of 0.95 g/L at 25’C in water and product has 7.3% Ca as ~30% calcium citrate with a use rate of 0.2-0.4 ml/L, as long as the bulk nutrient solution contains less than 190 Ca from other sources any calcium citrate that forms in situ should remain in solution.
A solution of 0.006M HCl is strong enough to dissolve 3.5% calcium citrate, and if I’m correct, the product contains ~30.26% calcium citrate. I tried to find solubility curves for calcium citrate in HCl by molarity of HCl (1M, 2M, and 3M), but I didn’t find anything. Calcium citrate is freely soluble in 3M HCl, so I’m guessing 1M HCl could dissolve ~30% calcium citrate.
It will remain a mystery without testing or info from the mfg. But it’s an interesting product and fun to play detective. ![]()
Foliar appliciation of inorganic or organic S is worth consdiering.
If you want to read more there are many studies and books on this topic. For example:
The fate of excess sulfur in higher plants (1982)
Sulfur Homeostasis in Plants (2020)
Marschner’s Mineral Nutrition of Higher Plants (Third Edition) (full text, click “GET” and search for sulfate)
I agree. It’s interesting for a few reasons, including increasing nitrogen uptake. Thiosulfate is metabolized and assimilated by a different pathway than sulfate. But for dicots (at least Arabidopsis), thiosulfate may be an inferior sulfur source (reduced biomass) compared to sulfate with >300 uM sulfur in solution. Plus, thiosulfate neutralized chlorine compounds, including hypochlorite and hypochlorous acid, so it probably wouldn’t be useful for growers who use chlorine compounds.
Organic sulfur is another route, for example, cysteine and glutathione. But without a sterile solution, the amnio acids probably would be microbially degraded. And there is uptake inhibition/antagonism of sulfate by sulfur-rich amino acids.
If you haven’t seen it, this is an interesting paper:
How was the smell/taste on the finished product after adding the epsom?
I never made it to harvest with Jacks 12-4-16 RO and Epsom, the buds smelled so weird that I was scared it would be unsellable, so I changed it to 321. There are other differences also, Jacks 12-4-16 RO is low P as well, I don’t know the reason for the strange lack of smell or chemical smell on it.
Did you end up trying out the vidawool? I’m getting ready to order my slabs for my first flower run at my facility and i want to use slabs that are pre cut, at home I normally use the grodan slabs but cutting 14 slabs vs 170 is a big difference.
I can get the VidaWool from one of my wholesalers that’s why I’m wanting to try it out.
Not yet, but after reading my posts on VidaWool in this thread, the market development leader for VidaWool DM’d me a while ago, offering to chat. He’s a member of this site. I haven’t had a chance to follow up yet, but I will in the next two weeks.
He was very friendly and offered to answer any questions I had. And he even said their team of scientists would conduct tests on the VidaWool if he couldn’t answer some of my questions regarding its physiochemical properties.
I can say just from the DM that VidaWool has impressed me. While Grodan has been open with me and willing to share info, VidaWool is going beyond by offering to have their in-house lab test their products.
hey @Medicine.grower are you still roughly following dankemhunters athena scheduel?.
its just i noticed athena have updated the webpage and they now recomend altering the ratios of core to bloom on week 4 and week 7 of flower.
they recomend the standard 3/5 ratio up untill week 4, then from week 4 to 7 the ratio is 3/7, then from week 7 the ratio is 2/8.
im not sure if this info was there on the old page and i just missed it or if this is new?.
if it is old info did you follow it and alter the ratios?, i dont remember you mentioning it in your thread?.
if this is new info do you intend to follow it in future runs?.
I see that he posted that they updated, I just had a chance to look it over, it’s the first I’ve heard of this, I had messaged him about a month or so ago because someone had asked my about changing ratios at the end of flower and he had said that he kept them the same so this must be new.
@emdub27 have you heard of cutting almost half the calcium nitrate out the last few weeks?
How much do your leaves fade at harvest? Flowers have very little calcium content compared to leaves, but have nitrogen content similar to leaves. In general I’m always trying to supply just enough N to control when the fade happens. It’s really going to depend on how much N the plant started flower with, if it starts with 5.5%N, you’ll be struggling to get the N out of the plant pretty much all the way through flower and dropping CaNO3 out would be prudent.
If we are trying to grow flowers and not leaves after week 3 of flower, then in theory we should formulate according to flower and not leaf composition, adjusting the nutrients accordingly. This approach works really well in my experience, at least to maximize yield. If we’re talking about the quality of the flower, then we also need to consider the nitrogen to carbon balance of the plant.
I’m assuming that can only be done through tissue analysis?
Also, what’s a good starting point for N for a flower formula? If running say around 160 No3, is 100N a good starting point for flower or, is that going to be dictated by tissue samples? Also if dropping CaNo3 in flower, where does the Ca come from then? Ca Acetate? If I run close to a 1:1 N:Ca ratio, should I be dropping the Ca along with the N in flower? Keeping them close to a 1:1? Sorry for all the questions. ![]()
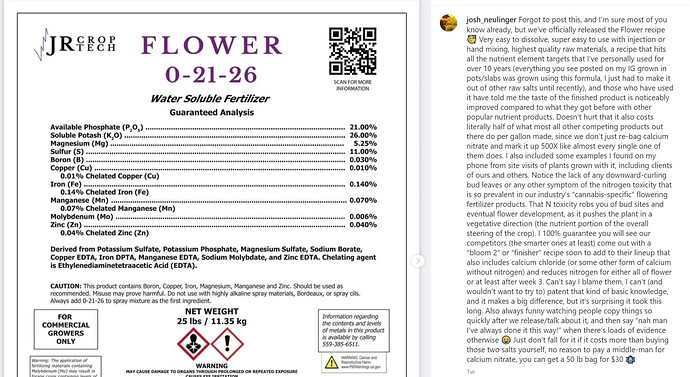
I was wondering what some of you nutrient pros thought about this new offering I saw on IG.
I don’t know much about the topic of nutrient analysis but I am becoming very interested in the things you all are talking about.
I love the idea of using tissue analysis and other forms of analysis to guide the nutrient program. Things have certainly come a long way from 10 years ago when I was last growing and it was basically all guessing.
Here’s a couple interesting quotes from the caption.
“…those who have used it have told me the taste of the finished product is noticeably improved compared to what they got before with other popular nutrient products. Doesn’t hurt that it also costs literally half of what most all other competing products out there do per gallon made, since we don’t just re-bag calcium nitrate and mark it up 500X like almost every single one of them does.”
" I 100% guarantee you will see our competitors (the smarter ones at least) come out with a “bloom 2” or “finisher” recipe soon to add to their lineup that also includes calcium chloride (or some other form of calcium without nitrogen) and reduces nitrogen for either all of flower or at least after week 3."
@Tech1145 The cheapest way to approach nutrition at a medium to large scale will always be to mix your own nutrients. It is the only way you can get to less than 2c per gallon of final nutrient solution (that I could find at least).
About claims, almost all nutrient companies will make some sort of claim about their product being “better than their competitors” and that “people who tried it got better results”. However, you seldom see evidence in proper trials of any of these matters. I always automatically discard claims that are provided without evidence when they are made by someone trying to sell me something.
Light, irrigation and CO2 are necessary contributors to yield and quality. If any of those fail or change then any comparison between nutrients is irrelevant. Plant research is specially hard due to this fact.
Hey I have been reading over some of your posts recently and I’m curious to know, what is your educational background?
Now that extraction is imploding I’m thinking about getting back into hydroponic growing because that’s what initially got me interested in the cannabis scene.
This will sound like a completely newb-ish question but how did you learn about using tissue analysis and knowing what the readings should be for cannabis and how to change them by altering the feed?
How do you know so much about combining different salts and what will be antagonistic with what?
I do have some basic first semester organic chem knowledge and I have seen the Mulder’s Chart so it’s not completely foreign to me.
Do you have any recommendations on how can I get into all this and learn more about it?
Do you see a promising future in this type of work (not necessarily Cannabis focused)?
I really love the idea of using this type of data-centric approach to cultivation. It’s something that didn’t seem possible for cannabis last time I was growing and I always felt like I was just guessing about stuff.
Thanks.
My degree is in production horticulture. Since then I have re-educated myself on plant nutrition, mainly following the work of Albrecht, Reams and Tainio.
It is important to note that at this point in time there is no perfect tissue sufficiency or DRIS data published for cannabis. All tissue sufficiency studies to date have been averages of crops tested by one agency, just because it’s average or normal doesn’t mean it’s correct.
I’ve developed my continually evolving targets over the years by first identifying that I was growing way better pot in soil than soilless using specialty nutrients available at the time. I did tissue analysis on those soil grown plants that were of higher quality to my customers and I. What I found was drastically different ratios compared to my hydro grown crops. Around this time is when I started looking into using a brix refractometer. Just to be clear brix is mostly considered pseudoscience by the majority of professionals in my field. But I noticed that the “better” soil grown crops were high brix. Albrecht and Reams heavily focus on soil management and ratios to grow a high brix crop, not very useful when growing soilless. Tainio focused on nutritional ratios within a plant, his findings are more useful for soilless culture. One of the key ones is that you can’t grow high brix unless potassium is higher than nitrogen.
I’ve been growing weed as a sole source of income since 2006, and got my degree in 2008. My knowledge of antagonisms and synergism specifically in cannabis has been built over that time.
The upcoming USU class would be a good place to start to understand broad concepts and the underlying reasons behind them. I do not know of any single source to learn about brix, just read and apply every publication you can get from Albrecht, Reams, and Tainio.
There will always be a future in agronomy and horticulture. It all depends on your expectations, average salary for someone with my background working full time in a tomato house is probably about $60k. To get to the $100k+ range you need to put in time with a big company like Heinz or do freelance work as a consultant. With the retraction in cannabis, I can be what many people consider expensive, so I do most stuff based on contracts where I only get paid if a clear performance target is met.
Thanks for the reading recommendations!
I just got contracted to put together a facility. We are going with FOHSE, Agnetix or Fluence fixtures, Meter Group or Agrowtek sensor logging, Cultivation Connect smart analytics, testing at New Age Labs and Athena nutes.
I wish they would spring for someone like an on-staff agronomist that could mix nutes, but they need entry level employees that can follow a feed schedule.