Go checkout further up in the thread on Dec 21st.
Nice glad to see this is charted territory.
Have you been running this replacement? If so how’d it go?
With straight STEM I’ve always seen issues, most commonly low copper. Use something else or amend it.
AFAIK no one here has been using a core replacement. We just discussed it.
No I haven’t, I’m still just running the core.
If anyone has any Athena Grow they want to send me, I’d be glad to pay for the analysis and share it here. I just need 5 grams or so. Send me a DM.
I can’t bear buying another 25 lb bag of fertilizer to potentially join my stable of unused ferts. ![]()
My veg on Core+Bloom in Sunshine Mix #4 is looking pretty good. EC 2.0 doesn’t seem like enough though and I have bumped up the EC. Plants have lighter new growth and some sort of deficiency, causing light chlorosis between the ribs on the leaves.
I’m not sure about in sunshine mix but in coco or rockwool I start at 3.0ec in veg and work my way down as pwec starts to stack in the medium. Right now I have mom’s in coco at day 46 of veg and I’m at 2.6ec. I had to top them again a few days ago to keep them out of the lights they were about 6.5 ft tall.
Also I can maybe get analysis of the grow next week, I’ve got a stock tank mixed of it now I could get a sample from.
DMed ![]()
What is your target pwec?
In veg I keep the sat pwec at 4-6. That would be my p2 pwec. Any lower then 4 I’ll start to see purple stems.
Is p2 the only stage youre concerned with pwec? Ive heard of pwec getting close to 50 in p1 without damaging plants ![]()
Whats your target p2 pwec in the heaviest part of bulking?
Yes and no, during stretch you will see pwec spike high at the end of p3 before they get p1. I never usually see high 50 but there’s for sure a big spike when getting around 25% dry back.
During bulking after stretch most strains I will steer vegative and still aim for the 4-6 sat pwec.
Then switch back to generative the last 2 weeks before harvest.
awesome thanks
Posting this here as it is new to me, thought others might enjoy it. I can say that Jacks 12-4-16 RO makes plants smell like nothing or even unpleasant and it has very low sulfur when used with Epsom as they recommend. The plants grow awesome on it though.
How was the smell/taste on the finished product after adding the epsom?
I would also like to point out that sulfur uptake in cannabis is saturated quite fast at a pH of 5.5-6. At this pH you will almost never reach S in leaf tissue above 0.4%, even if you try feeding 200ppm of S, the plants will often not uptake 20% of that and it will only build up in media.
So low S in the nutrient solution is almost certainly not why some formulations end up with poor smell. It might be correlated to it, but it is not the causal factor. I have seen great smelling plants grown with 40ppm of S, the media pH is managed to maximize uptake.
S uptake and S in solution are too entirely different things given how S transport works.
Will bringing the pH up to about 6.2 allow for more uptake of S?
You would probably have to go higher than 6.2 to see much higher uptake, at that point you 're also going to want to start using eddha chelates. The uptake limiting factor on S is why I don’t mind running it high. IME it takes obscene amounts to cause any real antagonism.
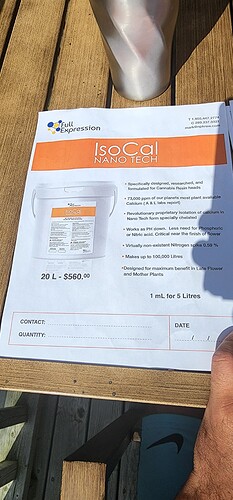
Have any of you guys seen this before? I was at a growers meet up last week and a rep from npk raw was there promoting this.
It has 7.3% Ca by weight with minimal N (~0.002%), K (0.1%), and Na (~0.22%). They tested for everthing except Cl (![]() ). And they claim it’s in “nano tech form,” reduces pH, and is chelated.
). And they claim it’s in “nano tech form,” reduces pH, and is chelated.
So, my guess is: Calcium citrate (tricalcium dicitrate tetrahydrate) in dilute hydrochloric acid. (or a different chelated source of Ca in HCl)
They probably used the term “nano tech” for marketing or used micronized calcium citrate crystals (powder) and took poetic liberties when defining “nano.” And I assume they avoided testing for Cl to try and protect IP, or they didn’t want to show a large amount of chloride because it would scare growers.
Calcium citrate solubility is only 0.95 g/L (950 ppm) in water at 25’C, but it’s freely soluble in 3M hydrochloric acid. I found assay data on a ~3.5% w/w solution of calcium citrate in 0.06M HCl, which contains ~0.84% Ca based on the formula for calcium citrate (Ca3(C6H5O7)2). So, I assume they can make a solution of 30.26% calcium citrate in 1M HCl.
The density of 1M HCl is 1.01636 at 20’C, and with ~30.26% calcium citrate, the density of IsoCal is probably around 1.3-1.32 g/mL.
If so, adding 1-2 mL of IsoCal per 5 L converts to ~0.26-0.52 grams per L. Therefore, 1-2 mL per 5 L would add around 19 to 38 ppm Ca to the nutrient solution with a pH addition of ~3.4 (0.2 mL of 1M HCl/L) to ~3.7 (0.4 mL of 1M HCl/L).
If they used 3M HCl (pH -0.48) instead of 1M HCl and assuming the density of IsoCal is ~1.355 g/mL, dosing 1-2 mL per 5 L would add around 19.8 to 39.6 ppm Ca with a pH addition ~2.92 to ~3.22.
The pH additions I listed are derived from 1M HCl (pH 0.00), and 3M HCl (pH -0.48). They don’t account for the neutralization of HCl by the chelator. So, the actual pH additions are probably higher than what I listed.
950 ppm calcium citrate (its water solubility at 25’C) contains 229.16 ppm Ca. So, as long as the nutrient solution has less than ~190 ppm Ca from other sources (like calcium nitrate), adding the recommended dose rate of IsoCal should avoid the perception of calcium citrate.
If you want, you could calculate the amount of Cl added based on my gusses and the dose rate of 0.2-0.4 mL per L.
What do you think, @danielfp? Is this a reasonable guess? Did I make any errors in the calculations?
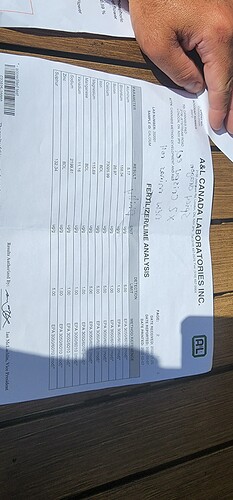
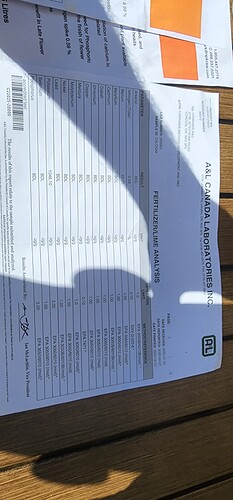
I don’t think you can say that this is calcium citrate. It could be any number of different things. Given the vague description of the product, all we know for sure is that it is a soluble calcium source that is not calcium nitrate. However without analysis we don’t know if the Ca is actually chelated, if there is any sort of nano structure or if it is just something much simpler (for example just Ca chloride in hydrochloric acid). It could also contain no chloride, there are also some potential formulations with this composition that contain no chloride.
Also from the bucket type container it would seem this product is a solid, not a liquid?
From your calculations, the only one I see would be problematic is your estimation of density, since the volume taken up by the solids changes a lot when they are dissolved. It is really hard to estimate what the density of a solution would be like, unless you have some insights into that relationship (for example experimental points of density vs concentration).
To better characterize this product, you would need deeper analysis. For starters a total carbon and chloride analysis might help you gauge the types and nature of the organics being used, but much more comprehensive tests would be needed for a more thorough characterization. If there are nano structures, then the effort to characterize those would be even more involved.
I don’t know if this would be worth the effort just to figure out a Ca containing product.