![]()
So you’re saying @beaker and @circus_animal had it right (or damn near)?!?
![]()
So you’re saying @beaker and @circus_animal had it right (or damn near)?!?
I totally forgot about that part!
I read all the procedures mentioning to pH balance otherwise the distillate would turn purple. Knowing that and religiously pH balanced this before solvent recovery. The first batch went purple. I pH balanced it again, same problem. Then I said fuck it and balanced the pH to 5 and it still turned purple. That’s what made me investigate magsil more. Thanks for bringing that up, that was an important detail
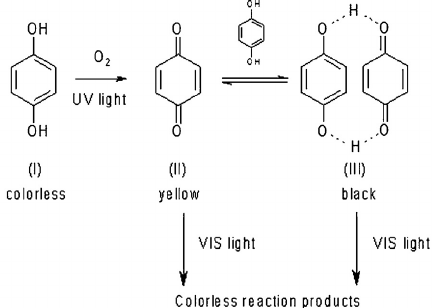
I actually made a crude mechanism for this and I think the oxidation reaction consumes oxygen gives off water and the reverse reaction does the opposite. I remember the purple color disappearing during distillation. If anyone has an FTIR, I bet a water formation peak would show up, though there may be a lot of noise compared to the signal since these colors are so strong the quinone could be in ppm levels.
I believe the vivid colors likely come from phenol-quinone coupling. The quinone may be less stable upon time, and further degrades to a yellowish compound under action of O2 and/or UVs.