The short answer is: yes.
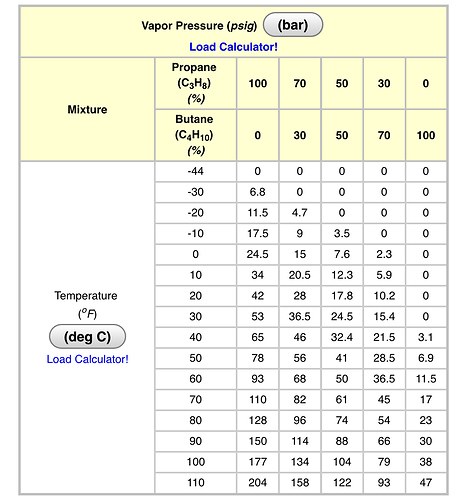
At a temperature of 110F (which is really too high of a temperature to operate at if you’d like to retain terps), the pressure of 100% n-Butane is 47psig (the g indicates what a gauge on a closed system would register).
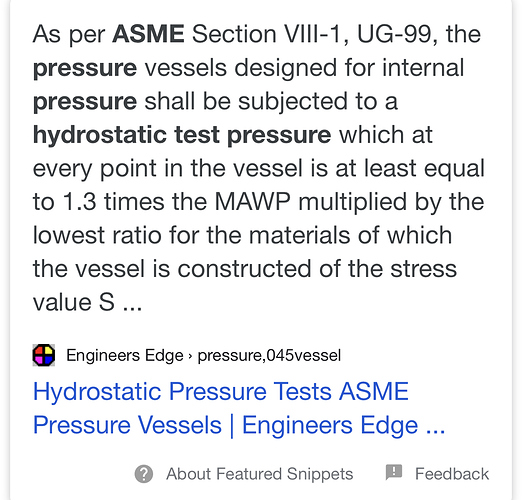
Per ASME pressure vessel code, a closed vessel should be able to hold 1.3x its pressure rating meaning a 100psi system should withstand 130psig. Which is about 36% of what your max pressure should be.
There are, however, some caveats:
you should be aware that the addition of nitrogen to the system can lead to pressures above safe levels…this is ESPECIALLY true if you are using a pump—please do not use a hydrocarbon recovery pump to pump nitrogen…they are to compress LPGs ONLY.
Pressure tests should be conducted after each assembly/disassembly.
Utilize volume and weight to ensure you’re not overfilling your system. It is better that you don’t fill columns and definitely not solvent tanks to the top. Leave some room.