You do realize that says its not as psychoactive as D9 but works just as good as d9 as an anti emetic
They add new chemicals every year to the list of chemicals covered under the federal analogue act.
The analogue act is part of the CSA which doesn’t apply to hemp though
Even the FDA stated this
You do realize it still gets you subjectively high like d9 right? And thats what they give a fuck about.
I’m willing to bet some money that they will move mountains to make d8 illegal, if its truly not defined as such right now.
§811. Authority and criteria for classification of substances
(a) Rules and regulations of Attorney General; hearing
The Attorney General shall apply the provisions of this subchapter to the controlled substances listed in the schedules established by section 812 of this title and to any other drug or other substance added to such schedules under this subchapter. Except as provided in subsections (d) and (e) of this section, the Attorney General may by rule—
(1) add to such a schedule or transfer between such schedules any drug or other substance if he—
(A) finds that such drug or other substance has a potential for abuse, and
(B) makes with respect to such drug or other substance the findings prescribed by subsection (b) of section 812 of this title for the schedule in which such drug is to be placed; or
(2) remove any drug or other substance from the schedules if he finds that the drug or other substance does not meet the requirements for inclusion in any schedule.
Rules of the Attorney General under this subsection shall be made on the record after opportunity for a hearing pursuant to the rulemaking procedures prescribed by subchapter II of chapter 5 of title 5. Proceedings for the issuance, amendment, or repeal of such rules may be initiated by the Attorney General (1) on his own motion, (2) at the request of the Secretary, or (3) on the petition of any interested party.
(b) Evaluation of drugs and other substances
The Attorney General shall, before initiating proceedings under subsection (a) of this section to control a drug or other substance or to remove a drug or other substance entirely from the schedules, and after gathering the necessary data, request from the Secretary a scientific and medical evaluation, and his recommendations, as to whether such drug or other substance should be so controlled or removed as a controlled substance. In making such evaluation and recommendations, the Secretary shall consider the factors listed in paragraphs (2), (3), (6), (7), and (8) of subsection (c) of this section and any scientific or medical considerations involved in paragraphs (1), (4), and (5) of such subsection. The recommendations of the Secretary shall include recommendations with respect to the appropriate schedule, if any, under which such drug or other substance should be listed. The evaluation and the recommendations of the Secretary shall be made in writing and submitted to the Attorney General within a reasonable time. The recommendations of the Secretary to the Attorney General shall be binding on the Attorney General as to such scientific and medical matters, and if the Secretary recommends that a drug or other substance not be controlled, the Attorney General shall not control the drug or other substance. If the Attorney General determines that these facts and all other relevant data constitute substantial evidence of potential for abuse such as to warrant control or substantial evidence that the drug or other substance should be removed entirely from the schedules, he shall initiate proceedings for control or removal, as the case may be, under subsection (a) of this section.
(c) Factors determinative of control or removal from schedules
In making any finding under subsection (a) of this section or under subsection (b) of section 812 of this title, the Attorney General shall consider the following factors with respect to each drug or other substance proposed to be controlled or removed from the schedules:
(1) Its actual or relative potential for abuse.
(2) Scientific evidence of its pharmacological effect, if known.
(3) The state of current scientific knowledge regarding the drug or other substance.
(4) Its history and current pattern of abuse.
(5) The scope, duration, and significance of abuse.
(6) What, if any, risk there is to the public health.
(7) Its psychic or physiological dependence liability.
(8) Whether the substance is an immediate precursor of a substance already controlled under this subchapter.
(h) Temporary scheduling to avoid imminent hazards to public safety
(1) If the Attorney General finds that the scheduling of a substance in schedule I on a temporary basis is necessary to avoid an imminent hazard to the public safety, he may, by order and without regard to the requirements of subsection (b) of this section relating to the Secretary of Health and Human Services, schedule such substance in schedule I if the substance is not listed in any other schedule in section 812 of this title or if no exemption or approval is in effect for the substance under section 505 of the Federal Food, Drug, and Cosmetic Act [21 U.S.C. 355]. Such an order may not be issued before the expiration of thirty days from—
(A) the date of the publication by the Attorney General of a notice in the Federal Register of the intention to issue such order and the grounds upon which such order is to be issued, and
(B) the date the Attorney General has transmitted the notice required by paragraph (4).
(2) The scheduling of a substance under this subsection shall expire at the end of 2 years from the date of the issuance of the order scheduling such substance, except that the Attorney General may, during the pendency of proceedings under subsection (a)(1) of this section with respect to the substance, extend the temporary scheduling for up to 1 year.
(3) When issuing an order under paragraph (1), the Attorney General shall be required to consider, with respect to the finding of an imminent hazard to the public safety, only those factors set forth in paragraphs (4), (5), and (6) of subsection (c) of this section, including actual abuse, diversion from legitimate channels, and clandestine importation, manufacture, or distribution.
(4) The Attorney General shall transmit notice of an order proposed to be issued under paragraph (1) to the Secretary of Health and Human Services. In issuing an order under paragraph (1), the Attorney General shall take into consideration any comments submitted by the Secretary in response to a notice transmitted pursuant to this paragraph.
(5) An order issued under paragraph (1) with respect to a substance shall be vacated upon the conclusion of a subsequent rulemaking proceeding initiated under subsection (a) of this section with respect to such substance.
(6) An order issued under paragraph (1) is not subject to judicial review.
(h)1 is of particular note
if we look at 812 under the general heading “schedule 1” we see:
(c) Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation, which contains any quantity of the following hallucinogenic substances, or which contains any of their salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation:
- Tetrahydrocannabinols.
Cannabimimetics are named in a different category, d8 would just fall under tetrahydrocannabinols
They can’t just outlaw whatever they want, they still have to show several things
Actual abuse - this means they have to show harm is being done by usage of this drug
GL with that with D8
The whole point of this is theres never been binding studies done to show how psychoactive D8 actually is
For them to outlaw it they’ll need that
and like I stated in my edit, the CSA only lists Tetrahydrocannabinols, not D9 THC, so this doesn’t even require interpretation as something new - it’s already specified as a controlled substance
You’ve got 3 lawyers tho.
Kinda shit the bed on the quote but u get what I’m sayin
You know how hard it would be for you to prove either of these?
Every cbd site I’ve ever seen that sells cbd let alone D8 requires ID verification and COAs (i know this as I’ve been trying to get square for my site) proving widespread abuse of this over the internet wouldn’t be easy because you’d have to have legal COAs to even get the POS terminal
So you can prove D8 is addicing?
Goodluck!
So D8 comes from seeds and mature stalk?
The problem is most D8 online is hot for D9 so they’re noncompliant by the hemp law, it’s just they choose labs that tell them they are compliant.
If your hemp derived D8 isn’t hemp, you’ve got MJ.
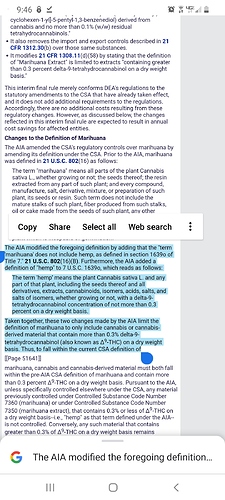
The AIA does not impact the control status of synthetically derived tetrahydrocannabinols (for Controlled Substance Code Number 7370) because the statutory definition of “hemp” is limited to materials that are derived from the plant Cannabis sativa L. For synthetically derived tetrahydrocannabinols, the concentration of Δ9-THC is not a determining factor in whether the material is a controlled substance. All synthetically derived tetrahydrocannabinols remain schedule I controlled substances.
This rulemaking is modifying 21 CFR 1308.11(d)(31) to reflect this statutory change. By this rulemaking, 21 CFR 1308.11(d)(31) is being modified via the addition of subsection (ii), which reads:
“Tetrahydrocannabinols does not include any material, compound, mixture, or preparation that falls within the definition of hemp set forth in 7 U.S.C. 1639o.”
7 U.S.C. 1639o
(1)Hemp
The term “hemp” means the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis.
I can see where your conclusion comes from based on the language of this one little excerpt, but given the fact that
A.) it’s specifically listed on the DEA’s website as schedule 1, and the AG has unilateral authority to place it there
B.) no one has provably compliant d8(I’d imagine the alphabet boys have a plethora of non-compliant samples from plenty of people on here and elsewhere)
C.) Chevron deference is in favor of the DEA
I’d say you’re fighting a losing battle. Even if you escape conviction(assuming you’re prosecuted), there’s no way to walk from this cleanly.
I obviously don’t hope the government decides to intervene(aside from quality standards/urgent health and safety issues - it’s been proven that self-regulation is a fucking myth), but operating as openly as many doing this have… Well, I don’t have high hopes if you ever see a courtroom
I agree with this, im talking about ppl who have D8 thats not hot
Wrong, cannabis is a schedule 1 specifically
D8 can’t be as there’s no binding studies done
Theres synthesis on this site for compliant D8 that I’ve posted myself, its not impossible to make compliant D8
Chevron Deference does not apply as they found it wasn’t congresses intention to schedule hemp as psychoactive
DEA-2018-0001-0003.pdf (344.0 KB)
I encourage you to take a dive into this PDF, this is the auction they used to banned all fentanyl analogies.
If the DEA thought they could schedule D8 in a schedule 1 temporarily they’d do it
They can’t as 1 it has medicinal benefits
And
2 ppl aren’t dying from it
GL scheduling the only medicine we’ve ever found that prevents puking in 100% chemo therapy patients
So even still, the party ends the moment they have a study that confirms what we already know about d8?