based on the picture, I’d say @PSam is developing with fast blue salts.
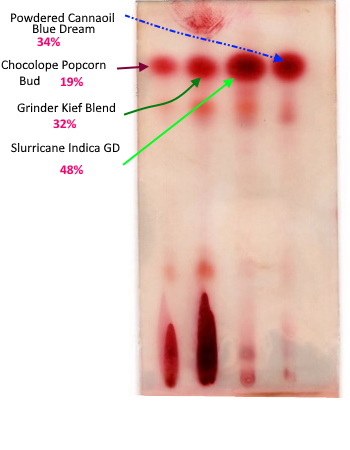
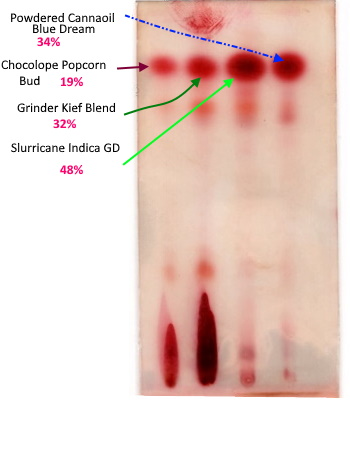
Oh, I’m extremely confident in my decarb. That spot is raw plant material and should be chock full of THCa. Those first 2 lanes are raw plant/kief. The last 2 are my decarbed products, etoh tincture and coconut oil powder. Those decarbs are perfect. I’ve spent a lot of time and money on lab tests getting that figured out.
If those other lanes had shown a THCa streak like those raw ones then I would really be freaking out. ![]()
You know, I’m not really sure. I think the dye is that blue one @cyclopath mentioned. I bought a kit and haven’t been able to find that info on their site. I have no clue about the solvent. I’m hoping to be able to learn what those are and then just restock on my own. Just the few brief interactions here have focused my insights already but I have a long way to go.
My “chamber” is an old shoebox I use for spraying. I’ll probably start dipping instead, though. ![]()
If there is THCA in there, you cannot use your 19% thc COA to label the THC spot on your plate 19% THC is my point.
The folks that gave you the COA did math on the THCA to come up with that number.
I’m aware of that and I’m not trying to pass it off as gospel. The dispensary numbers need to be taken with a grain of salt. I’ve been a VIP client of C4 labs here for several years and have a better than average understanding of the processes for a layman.
I’m not trying to quantify the THC on those raw material samples and am happy with the total THC isomers, just like the labs. They do the math as you stated and just provide that number as a summary. The THC doesn’t get delineated from the THCa until you get into the actual analysis.
I found a guide for one of the kits that stated those acid ovals could be rounded out and then subtracted from the THC-or vice versa-if one wanted to quantify further. Another guide said to use 4 lanes for 1 sample and then subtract lane x from lane y. Who knows what the next one will say. Maybe I can bunch them all together and call it “TLC for Dummies” and make a fortune. Yeah, right. ![]()
I’m not able to easily hook up with the lab any more but I still have projects that I need some testing for so I’m trying this. I’m using it as a teaching tool. I have a dedicated following and I owe it to them to get things right.
So right now I’m doing my homework and trying to learn the best I can. You brought a couple of good points that I need to be sure of and maybe focus a little more on first even with my confidence. Those are basic principles and if I don’t have those correct from the start then everything following is in doubt. ![]()
Hi PSam, THC is calculated as 0.877*THCA. This is due to the loss of CO2 from THCA during decarbing (adjustment for change in molecular weight).
Total THC then is calculated as measured THC + 0.877*THCA.
As for decarbing, we do testing for local growers. While the decarbing is a pretty established technique, many samples we measure that are decarbed still contain a small percentage of acids - we use HPLC.
Thank you. I’ve actually been testing with C4 labs here for years and have decarbing pretty well understood and I’m familiar with the way the THC is calculated for the total. Many of my projects have been decarb studies and for those I’ve always used the individual values instead of the basic total.
Unfortunately, I no longer have my VIP discount at the lab nor the resources I used to have but I still like to take that extra step when verifying my projects. So I have been trying to learn the intricacies of TLC and how I can use it for my needs.
I was under the impression that I could quantify potency for extractions other than concentrates, things like tinctures, oils and other ingestibles, and was trying to figure out how to read the percentage template for those results in mg/ml instead of mg/g. This was the formula I was seeking but have since learned that I’m not going to get that detail from it. I can still see profiles and whether decarb was complete and can at least judge concentration.
I like to keep some acids in my decarbed product. Once you get past a certain percentage of conversion you start losing as much to degradation on the other end.
I guess I’m still going to need to use the lab if I want specific values but at least this TLC gives me a bit of consistency.
Thanks again for the feedback.
There’s a trick for using bromocresol green for evaluating decarb floating around here but it might not be what you’re looking for if you want only partial decarb.
You can certainly quantify cannabinoids with TLC. Within 10% m/m is pretty achievable and some have done a lot better than that.
Usually the issue with edibles and other forms is with the extraction of cannabinoids from the product and not the actual analytical method.
Lastly, if all you want is g/g to g/ml, just multiply your mass potency by the density of the product
I know that there are other dyes and solvents other than what came with my kit and I expect to learn about those as I progress but right now I’m just a dummy and have no clue to the materials I’m currently using. There’s not a lot of “how to” support from these companies so I’m having to piece it together.
It’s not that I particularly want to have acids left but I’m knowledgeable enough to realize that my kitchen oven isn’t going to give those kinds of results without going too far in the other direction. So seeing acids on the plate for a decarbed product isn’t a big thing. I don’t really expect pinpoint accuracy.
The only quantitative values I’ve seen from it is when I use plant material to find the percentage using a hot read. I have a template to compare that to. Unfortunately, that percentage template doesn’t convert for me although I can calculate concentrate percentages with it, as long as it’s on the mg/g scale.
I believe you have put your finger on my basic problem. I kind of thought that it had something to do with density but that’s all I suspected. I didn’t know the formula since the lab always did that for me. If they used the wrong scale then I’d just call and ask them to convert it for me. I never learned to do it myself.
I guess it’s time to buckle down if I’m ever going to conquer this and quit dreaming that I’ll just wake up and understand it all one day. Thanks again for your help.
And now you’ve really piqued my interest with this and given me more reason to delve into the search function. I’ll see if I can find it. ![]()
Yeah so searching bromocresol green will bring you to the easy button for evaluating when your decarb is done.
As far as quantifying with TLC, let’s pretend we’re only measuring the amount of d9 in a sample:
We start with a standard (this can be something with a known amount of d9, doesn’t need to be a legit CRM for our purposes). Let’s say it’s tested at 90%. We dissolve 100mg of that oil in 10g of solvent (apparently chloroform works well). If we spot this, we’ll have 90mg THC in 10000mg or 9% w/w.
What we’re looking at is the area of the spot developed. There are several web apps for scanning in pictures of your plates to measure the area of your spots.
If we then extract 1 gram of flower with 100 grams of chloroform and spot that, if the entire gram of flower dissolved we would have 1% THC, giving us a 10% larger spot than our standard. Accordingly, if we use a ratio of 100:1 in our extraction, a spot that is 110% of the area of our standard equates to 100% d9. 1/5 the area=20%.
FYI, the above numbers/ratios are for illustrative purposes, probably not good ratios for your method. Errors in the amount spotted account for the majority of TLC error. Precision is better if you adjust your standard spot to be similar in size to the spot of your analyte.
As soon as the results for bromocresol green appeared I knew that everything I knew about decarb just flew out the window. And I thought I knew all about it. Ha! I’m going to be following Alice down the rabbit hole on this one.
Thank you for that so much. That’s what I was looking for. I kind of find it amusing, though. What’s amusing to me is that just a few months ago I wouldn’t have a clue about what you just said but now I can understand it and know how to convert to my needs. You’ve given me an outline now to build from and I’m looking forward to the exercise. I’m learning more here than I ever did in college. ![]()
It’s in “tricks of the trade” thread…
Under my screen name
Great. Thank you very much.
big ass “oof” @ “none.” What are ya’ll doing in the fine chemical sector flying blind?
You know what happens when you fly blind? You crash and burn. I have no intentions of doing that and I hope no one else does, either. I sure hope “big ass oof @none” gets down safely. Personally, I’m just taxiing down the runway waiting for the visibility to clear. ![]()
This webinar definitely placed an interesting view on analytical testing from a USP and Ph Eur perspective. Supporting chapters were very useful.
Hi there,
Just following up on this post. For anyone doing in house cannabis analysis and using (or interested in using) a SRI GC, our model 310 MM tests marijuana for cannabinoids (THC, CBD, CBN, etc.), terpenes (pinene, myrcene, limonene, etc.), as well as residual solvents (butane, ethanol, etc.)
Please visit the YouTube channel to learn how to get started and to find answers to any questions regarding its use and maintenance. Just search “SRI GC Training” on YouTube or Google.
SRI’s US-based customer service is also happy to answer any questions on the phone (310-214-5092, from 7am-4pm PST) or on here as well.
Happy Friday to all.
-Stephanie