yeah, monkeys are notoriously error prone. some provably more so than others. machines are at least systematic (generally) in the errors they introduce ![]()
The way I look at it, at $80 for a calibrated hamilton syringe, you only have to reduce fucking up a little bit to pay that $400 off pretty quick.
I do wish I had a heated headspace sampler though
Sigh… dreams…
tell me I won’t build a heated sheetmetal box around the 8400 autosampler I have lol
I’m going to win the “kludgiest analytical chemist” award
I was thinking of heating the sample vial holder ![]()
How much volume is in your sample prep? So far I’m seeing mostly 40ml thereabouts being used. Assuming thats the case, for auto sampler, i guess you’d have to transfer to the sampler vials?
Currently 40ml. But only because that’s what came in the door, and it works…(GC has heated space for 12vials)
40ml vials is what I use for my GC as well. I actually use those 40ml vials for just about everything in the lab. Taking samples from our production chromatography runs, taking samples during extraction to test. You name it if it’s fluid it goes in those vials at some point. However when testing on our HPLC we transfer from those vials to our 2ml vials that fit in our auto sampler.
Do you use a filter for particulates when you do? What do you use for the transfer itself?
5ml syringe with a .22 um hydrophobic filter. I’ll clean and reuse the syringes and filters when it’s not a critical test. Otherwise it’s a lot of plastic waste.
All critical tests get a new syringe and filter.
This is exactly what i was concerned about. Having to dump a bunch of single use plastic just to run tests.
What do you clean them with? Acetone?
Usually ethanol or methanol.
For my QC testing folks out there, with all the talk of d8 contaminant/byproduct testing taking place here are some links for some reference materials that can be used.
| Analyte | CAS No. | Concentration |
|---|---|---|
| o-Cresol (2-Methylphenol) | 95-48-7 | 2000 µg/mL |
| m-Cresol (3-Methylphenol) | 108-39-4 | 2000 µg/mL |
| p-Cresol (4-Methylphenol) | 106-44-5 | 2000 µg/mL |
| 2,6-Dimethoxy-4-methylphenol | 6638-05-7 | 20000 µg/mL |
| 2,6-Dimethoxyphenol | 97-53-0 | 20000 µg/mL |
| 2-Methoxy-4-methylphenol | 93-51-6 | 2000 µg/mL |
| 2-Methylcatechol | 90-05-1 | 2000 µg/mL |
| Phenol | 108-95-2 | 2000 µg/mL |
| Components | CAS# | Concentration |
|---|---|---|
| Bis(2-Ethylhexyl)phthalate | 117-81-7 | 2000 µg/mL |
| Bis(2-methoxyethyl)phthalate | 117-82-8 | 2000 µg/mL |
| Bis(2-n-butoxyethyl)phthalate | 117-83-9 | 2000 µg/mL |
| Bis(4-methyl-2-pentyl)phthalate | 146-50-9 | 2000 µg/mL |
| Butylbenzyl phthalate | 85-68-7 | 2000 µg/mL |
| Diamyl phthalate | 131-18-0 | 2000 µg/mL |
| Dicyclohexyl phthalate | 84-61-7 | 2000 µg/mL |
| Diethyl phthalate | 84-66-2 | 2000 µg/mL |
| Diisobutyl phthalate | 84-69-5 | 2000 µg/mL |
| Dimethyl phthalate | 131-11-3 | 2000 µg/mL |
| Di-n-butyl phthalate | 84-74-2 | 2000 µg/mL |
| Di-n-hexyl phthalate | 84-75-3 | 2000 µg/mL |
| Di-n-octyl phthalate | 117-84-0 | 2000 µg/mL |
| Dinonyl phthalate | 84-76-4 | 2000 µg/mL |
| Hexyl 2-ethylhexyl phthalate | 75673-16-4 | 2000 µg/mL |
I could not find any Olivetol reference materials but since there should not be any, you can use the 95%+ as a qualitative peak.
@iontrap anything you would add/change?
**I am not affiliated with Emerald, just use them as they have a lot of stuff in one place = one shipment.
MS only?
FID should work as well. I have not personal looked for these compounds in conjunction with cannabinoids/matrix but I would imagine the separation would be easily achievable.
Olivetol is the only one I could not find an exact paper for, but I see no reason why you would have an issue with it.
Okay updates:
The GC now speaks with the computer so that’s nice! I have to replace a fan in the workstation and when I do I’ll post the ethernet card info as well as the OS version, driver version, and software version so people can replicate.
Still no love on the EFC, I seem unable to open any of the valves by jogging manually. Need more time to sort this one out.
New problem: I rewired the MS for 120v and plugged it in but I don’t have anything other than a “fuse OK” light showing. When the service switch is in the “off” position that light dims slightly. The “line OK” light does not come on, nor do any of the various voltage status lights. I suspect that the power board may not be in good condition. This is the part where I curse my luck for showing @tokesandtinkery that $200 MS because now I’ll be buying the $700 one to steal parts from lol (just kidding, I’m still happy he bought it). I may dig around inside with a meter first to see if there’s power going to the right places first
Let me know if you need me to take apart this one for some reference images
That was my problem with molecular biology…the only way to get the job done was to sacrifice large quantities of virgin polyethylene every day.
Okay so a little update time:
Fixed the EFC issue by just swapping the ribbon cable and one of the pencil filters (not sure which was the trick and I don’t care either lol).
The MS took a bit of work. It was formerly wired for 220 and I changed the switch on the turbo control board (before I had identified it as such) to 110 but still had no love from the status lights. So I tore the whole thing apart and found two more switches tucked between the PWA board and whatever is on top of that. Bing bam boom, software speaks to the hardware.
Next issue was getting the vacuum set: the E2M8 I scrapped together for it wasn’t making good vacuum so I had to rebuild that. Even still, I was only getting up to 10% on the turbo speed so I figured it was a roughing issue. Three oil changes and another rebuild to make sure I didn’t fuck something up before I realized the obvious: not having the column attached to the detector would make for a bit of trouble pulling vacuum now wouldn’t it lol.
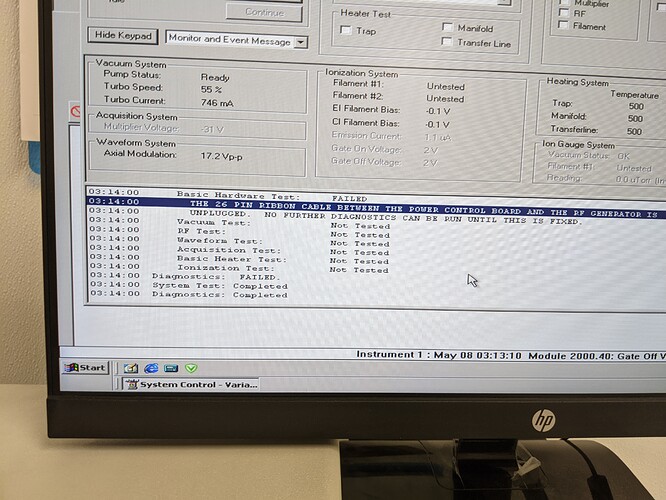
Rectified that problem and was able to get it to hit 55% and at least allow the software to start looking at data acquisition but I had no signal. Ran the diagnostic feature and got this:
Now, I did check the cable and didn’t see an issue but I haven’t had a chance to re-check it. I know that the RF boards are a weak point on these machines so I’m not super optimistic but oh well. Lots of progress and I might only be one $200 part away from being good to go.
I’m starting to write the prep procedures now and I could use a tip from those who know: what is a reasonable column load for one of these things? 10ng? 1ng?