You’re not pulling vacuum so I don’t see the need to get too crazy with the condensing power. Keep in mind when you use this setup during fisher esterification you’re working with volatiles like methanol, ethyl acetate, short chain carboxylic acids, etc. The reaction itself would not work if those were all gassing off past the condenser into the fume hood. That’s not to say I don’t have my share of dumb ideas to cool things more but those all require a complete disregard for the correct way of using lab glass, so I’ll keep those ideas in the DM’s for now.
Would you be continuously flushing the system with an inert gas then? I don’t see how there wouldn’t be any unwanted side reactions occurring with respect to the terpenes and other VOC’s if ambient air/oxygen isn’t taken into consideration.
Definitely will be reaching out to hear more *crazy ideas *
Anyone ever consider using a CO2 membrane to allow CO2 to escape the decarb chamber but nothing else?
Hi danny,
Im trying to decarb 2000 grams of oil @ 80c. I have 8L of headspace , i did the PRV calc and ill be under my rated 350 PSI rating. The only info i cant find is how long it will take at 80c v 90c v 100 c i cant seem to figure that out
can you help me
Determining that is beyond my math skills and knowledge. Best advice I can offer is you’ll know when you’re getting close to complete decarb when you get close to your predicted pressure at temp.
Ive read that terpenes can combine like Legos in a condensation reaction to form larger terps. I suspected this with distillate because the terpene fraction was always of aberrant qualities. Going from straight live to decarb i feel there is a noticable difference. Maybe if there was a liquid nitrogen cold finger in your reaction vessal to catch the thiols and other volatiles. when your finished bring entire vessel temp down very low, open and homogenize.
Nope. ![]()
I have done numerous pre and post decarb tests and all I see is an increase in terp concentration in about the same proportion to the weight lost from liberating CO2.
I think the LACY uses something like this.
I have a membrane but haven’t had time to mess with it.
@nikodank The rate of decarboxylation varies between different cannabinoid acids, and it increases with temperature logarithmically… I’ve seen a few graphs of decarboxylation “completeness” wrt temperature & time, but not a reliable rate equation.
was that terpenes broadly or did the ratio of the various terps stay the same?
Ratios stayed the same.
I have been doing similar calculations lately and have noticed some errors in this mathematical approach. It’s important to remember CO2 is not an ideal gas and has a different R value than the ideal gas constant, significantly different. SI units are important to use correctly.
Given the variables you stated, here is how I would approach the problem:
2kg of oil (average 75% THCA content - 1.5kg, assume 12.3% CO2 by mass - 184.5g CO2) 184.5g CO2 @ 44.01 g/mol = 4.192 mol
80C = 353.13 Kelvin
8L = 0.008 m3
PV=NRT (V=0.008 m3, N= 4.1922 mol, R= 118.92 J/kg K, T= 353.13 K)
P= 22,006,020.9 Pascal (1 Pascal = 0.000145 psi)
P= 3,191.725 psi
So, 3,000 psi is significantly higher than the 350 psi you arrived at. Please be careful in practice and always use a PRV.
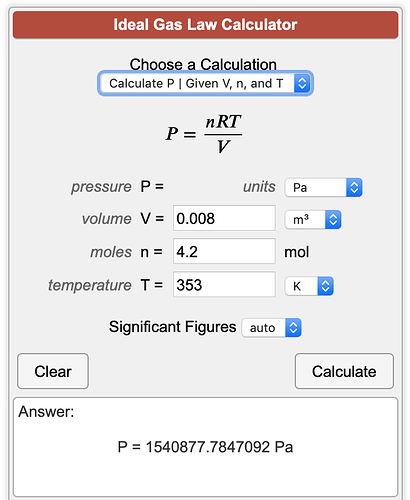
This calculator (Ideal Gas Law Calculator PV = nRT) although not using the CO2 specific R & molar volume values, gives a number that IS under @nikodank’s 350PSI PRV
the problem is that the ideal gas constant ( R= 8.314 J/kg K) and the specific gas constant for CO2 ( R= 188.92 J/kg K) will give dramatically different results when used in PV=NRT. It’s the difference between a bomb and not a bomb.
yep. that would do it!!
the molar volume is pretty close, and R is presented in so many different units I had not caught the HUGE difference there.
however, upon digging, I’m pretty sure the gas specific R values are only meant to be used when “n” is not. because Rspecific = R/molar mass
so how does 8.3/44 => 189?
44g => 0.044kg!!
==> 8.314/0.044 == 188.9
I’m out of my depth here.
can you perhaps clarify @TheGratefulPhil?
is CO2 “ideal”?
Or ideal-ish?!?
perhaps: Properties of Various Ideal Gases (at 300 K)
Mhmm I’ve just completed the test, it worked was about 24h and our pressure hit the number we were looking for. I’m doing test now to see if we are close to fully decarb but the attached looks like I am and I’m at the point of popping terps based on the big bubbles from what I learnt over here
Any feedback?
Anonymous, it never reached anywhere close to the max rating of vessel which is 350
Attachedhttps://vimeo.com/756209067
lick the ![]()
Poping terps under pressure means you reached the boiling point of that specific compound under pressure
Would like to know the pressure you where at and the temperature ?