I thought I would randomly bump this topic because of something I saw recently in the main CRC thread. It doesn’t fit this topic perfectly but oh well.
People were talking about whether or not their recovery pump could handle compressing the nitrogen gas used for vapor assisting in an extraction system instead of venting it to atmosphere.
Some people thought this should be avoided because it could damage the pump or cause a pressure spike.
I also saw a couple people incorrectly refer to nitrogen gas as incompressible. All gases are compressible, generally speaking.
Our atmosphere is mostly nitrogen and lots of pumps are used for air compressors.
Compressing nitrogen gas is generally ok and any type of positive displacement pump we use in extraction should be fine handling it. The pressure may increase slightly from the heat of compression, but it should not be anything too crazy in our situation.
I think a lot of the confusion here has to do with the fact that certain air gases are referred to as “non-condensable” and/or “non-compressible” interchangeably by different industries in and around the liquified air-gas industry.
This is very misleading, especially to say they are “non-condensable”.
The reason things like nitrogen, oxygen, argon and helium are referred to as non-compressible or non-condensable is because one of their properties called the critical temperature is below normal conditions we experience here on Earth.
A substance’s critical temperature is the temperature above which the gas cannot be liquefied by pressure alone.
Here are some critical temperatures:
Helium= -268C
Nitrogen= -147C
Argon= -122C
Oxygen= -119C
CO2= +31C
Propane= +97C
Butane= +152C
Taking nitrogen for example, what this means is that it is physically impossible to have liquid nitrogen at any temperature above -147C no matter the pressure you put on it.
Even if you had an unbreakable tank and applied a million PSI to the nitrogen vapor, if the temperature is -146.9C or warmer you will only get supercritical nitrogen, not an equilibrium with distinct pure liquid and vapor phases.
But if you lower the temperature to -147C you would only need to apply about 493psi or 34bar (the critical pressure) to get a vapor-liquid equilibrium with distinct phases.
These critical temperatures and their relationship to the boiling point of the given substance is determined by different molecular makeups and inter- and intra-molecular forces of each substance.
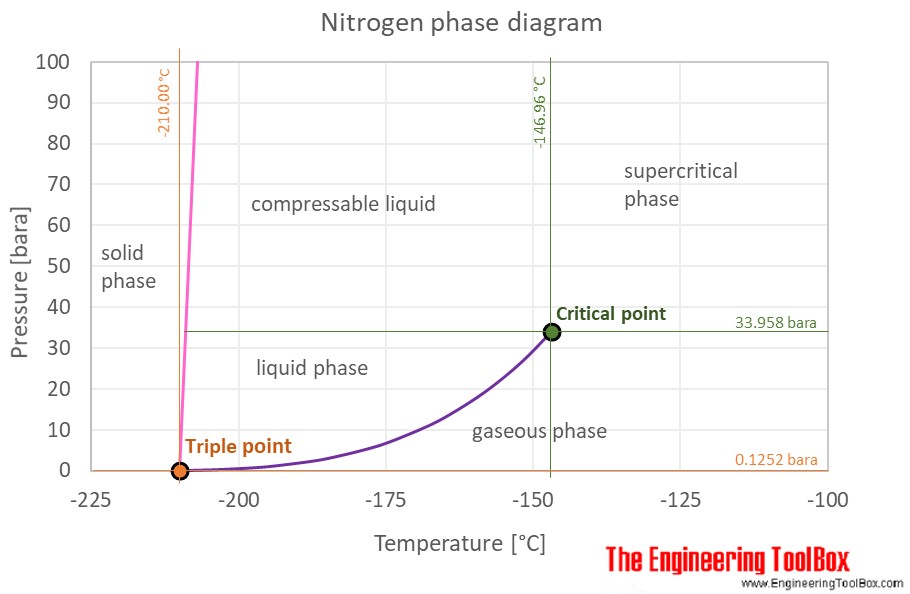
All of the relevant info is nicely displayed visually on a substance’s phase diagram, something every thermodymagician should know how to read.
Below is the diagram for nitrogen. The purple line is the boiling point curve which gives you the BP at different pressures. You’ll notice that the curve has a distinct “beginning” and “end” with the triple point and critical point. All substances are like this and the endpoints coincide with specific temperatures and pressures. You can see the critical point for nitrogen at -147C and 34bar. The graph makes it easy to see that you can’t have liquid nitrogen above -147C. You could still have a distinct gas phase and not supercritical above -147C if the pressure is below 34 bar.
Another issue I’ve seen out in public that relates to this topic is with propane vs natural gas (methane) with regard to home energy use.
These two gases are very commonly used as home energy sources but propane can be easily delivered and stored in tanks on site. Methane on the other hand must be piped in and cannot be easily stored in tanks at ambient temps like a propane tank.
This is because methane is a substance that also falls under the “non-compressible” umbrella with a critical temp of about -83C. Propane’s critical temp by contrast is +97C.
This is also why CNG vehicles have very strong tanks. There can’t be any liquid methane in the tank because the tanks are at ambient temp, well above the -83C critical temp. The best they can do is cram vapor in the tank up to several thousand psi. They have to use this high of pressure to be able to get enough vapor into the tank to get a decent driving range.
TL,DR: Certain air-gases are referred to as “non-condensable” and/or “non-compressible” because they can’t be liquefied by pressure alone at normal ambient conditions on Earth. They must be maintained at some lower temp to have distinct liquid and vapor phases and thus the liquid to vapor phase change we are interested in exploiting.





