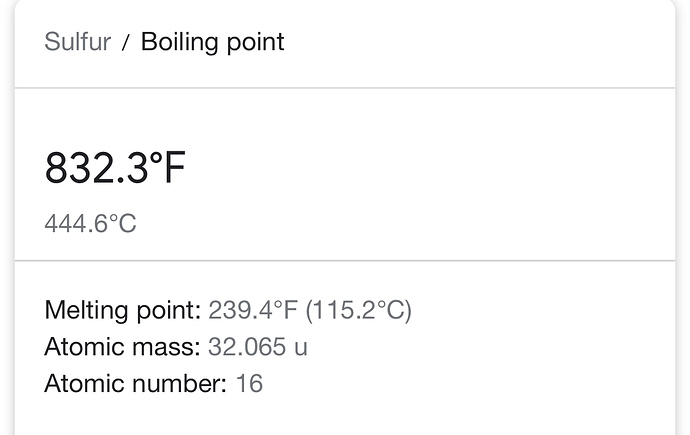
Pardon my ignorance, isn’t sulfur’s boiling point rather high?
Could you SPD everything else out, leaving behind crude with the sulfur?
Follow up question, is there any reason to think we couldn’t eliminate through column chromatography? Is the difficulty identifying sulfur in the fractions?
Problem being any residual heat would “reactivate” the sulfur into hydrogen sulfide gas ( @Dr_Jebril mentions this above)
That’s kinda what we are looking at now…
I’m lookin go at the Chalcogen family (which sulfur is in) and wondering if another element (like selenium) would work better. It falls between sulfur and another element in that family which makes it the “middle road” as a possible catalyst.
Edit: scratch that…easier to filter out but produces hydrogen selenide which is more toxic then hydrogen sulfide.
Much safer to use and much easier to filter out
It produce H2S again. Some escape and some H2S reacts with x S atoms to form a range H2Sx (x =3 to 6 in general), which seems to vaporize between 100 and 200°C, producing even more mess (recondensate easily as solid).
Distillation may also be a solution in fact. But needs a special approach for handling and separating well the volatilized sulfur. Needs a new design.
This may not make sense on any kind of large scale, but presumably we could use a method like his during column chromatography to identify fractions with elemental sulfur to re-filter?
To test for hydrogen sulfide it would work well…
Not sure about the residuals left after the hydrogen sulfide is “degassed”. Referring to the elemental sulfides left over in a solid form.
What about packing a head with copper scrubbers? Or, would that let the elemental sulfides pass through still?
It’s used a lot in distilling of wines and alcohols.
It does seem to reduce the sulfur content, but I don’t think it eliminates it completely. Have not done any testing
Or copper flakes in the boiling flask after the reaction once cooled …then LLE of Potassium Chloride, H2O, and Heptane followed by rotovaping with more copper flakes in the flask, filtered over a bed of AC and bentonite, then distilled with more flakes in the boiling flask and copper scrubbers packed in the head?
A bit overkill? ![]()
![]()
![]()
all pushed through a .5 micron copper scrubbie to polish it all off ![]()
I mean…
Look at the extent of filtration we already go through
Why not? ![]()
![]()
![]()
![]()
Anything of that matter in the boiling flask will promote further reactions…
The vapor scrubbing sound more and more like a smart move.
Could be simply made of a heated column in the head, where the vapors would be reduced to elemental sulfur and trapped there a solid. If made from clay, that scrubber would then be easily regenerated by re-oxidzing the clay. If made from copper, would be regenerated by acid treatment,
Still, it needs a special design, which maximizes the contact between clay and vapors, so it is all scrubbed in one pass.
Another key, would in fact to use a deficit of sulfur reactant, and go for an incomplete reaction (finishing it upon distillation). Or had another reactant in excess, that ensure that all sulfure gets eventually reduced and further vapoprized (as H2S and as polysulfides), and further trapped in the vapor trap.
Clay beads held back with copper wool?
Even if 2 passes would do it, I’d be happy.
So you think adding copper flakes to the reaction after its cooled would still cause other reactions? What about the original filtering with (in this order from top to bottom) copper flakes, AC, and then betonite?
I’d rather be known for a CLEAN product then a “pure” product (with hidden shit that most don’t test for let alone understand).
https://pubs.acs.org/doi/pdf/10.1021/ie990717z
How about using sulphuric acid to remove H2S
This is how its done in big refineries im pretty sure
Clay or polymer beads?
Wonder what pour size would work best if we can use zeolites?
Did not expect to spend my morning diving down this rabbit hole ![]()
![]()
![]()
Hydrogen sulfide isn’t the issue…
It’s the elemental sulfides left over. Need a way to capture it back in its solid form.
I equate this as using AC to “remove” the color from chlorophyll back in the day.
Later, we find out it’s just removing color but the elemental chlorophyll remained.