Feel like this is misleading for a number of reasons…
-
Didn’t mention that in addition to increasing the temperature, you also raised the extraction pressure from 1800psi to 3000psi.
-
Since when is 1800psi at 40C (104F) the industry standard? At that pressure/temperature, cannabinoids have little to no solubility in CO2 (with the exception of CBD).
-
Only two test conditions presented with drastically different extraction parameters.
-
No data presented for Δ9 yield/concentration. While it’ll occur very slowly, 70C toasty enough to decarb the material, especially if there’s any hotspots in the extraction vessel.
-
Not sure why the Perrotin-Brunel thesis is referenced as it does not cover THCA solubility (to the best of my knowledge, that research team has never posted a study of acidic cannabinoid solubility in CO2).
My biggest issue with this study (aside from it presenting only two test conditions) stems from the fact that cannabinoid solubility in CO2 does not always increase at higher temperatures. To quote the Perrotin-Brunel paper referenced by this study:
Another explanation for the differences in CO2 solubility may arise from the differences in melting point. It can be noticed that the liquid cannabinoids (CBD and CBN at 334 K, and 9-THC at all temperatures) show lower solubility in supercritical CO2 compared to the solid cannabinoids (CBD and CBN at lower temperatures, and CBG at all temperatures). This is consistent with the previous observation that melting results in a lower solubility of CBD and CBN in CO2 at higher temperatures.
The cross-over pressure of the different cannabinoids increases in the order of: CBN<CBD< CBG <9-THC. Interestingly enough, this shows the opposite trend with CO2 solubility i.e., the cannabinoid with the highest cross-over pressure has the lower solubility in CO2. This could also be the result of the melting point effect: the cannabinoid with the lowest melting point has the highest vapor pressure at given temperature. Because the crossing of solubility lines is a result of a trade-off between a density effect and a volatility effect, the cross-over pressure will be higher when the vapor pressure of a compound at given temperature is higher.
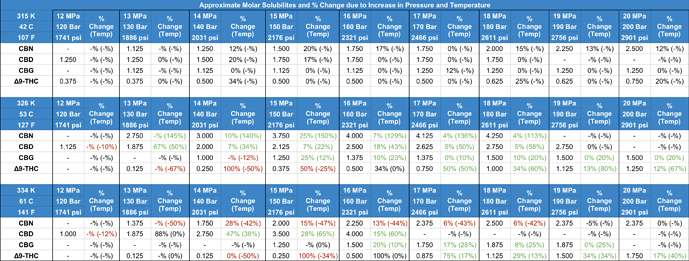
Unlike Δ9, THC-A solidifies at temperatures below 52C. Applying the same logic used by Perrotin-Brunel for non-acidic cannabinoids would suggest that a higher solubility of THC-A could be achieved by keeping the extraction temperature below the melting point and simply raising the extraction pressure to a reasonable value (2200 psi at 51C has a comparable CO2 density to 3000 psi at 70C). For instance, in the case of CBN, solubility actually decreases substantially when the temperature is raised above the melting point (note: not sure how the aromaticity factors into this, in general, cannabinoids with more C=C double bonds tend to have the highest solubility). I prepared a spreadsheet awhile back based on the data from the Perrotin-Brunel studies which shows the %change in solubility due to increasing the pressure or temperature (see image below).
Final note, I’ve heard a lot of claims regarding poor THC-A extraction efficiency relative to Δ9, but seen very little supporting data (if someone can point me towards a study on acidic cannabinoid solubility in CO2, please let me know). In fact, Supercritical carbon dioxide extraction of cannabinoids from Cannabissativa L. by Rovetto & Aieta seems to suggest the opposite, with THC-A being extracted quicker than Δ9 at 328K (130F) and 3000 psi (effect diminished at higher pressures).