Well, yesterder i do a mini test in some shit material, bad storage,material after a seeds harvest and some trim from tropicanna that was red dark asf.
I put 500mg of Ascorbic Acid in one liter of etoh stir until all disolve (take some minutes) and chill below -70( i be afreid that the acid precipitate at this temps but i dont see nothing in a short period time). Soak 3 to 5 minuts, only one pass, then i add another 500mg of AA (i want to try an exces) stir until all disolve. Roto vaped in vacum at 24 Celcius and a precipitate water this time with a pink color, i see this color before in others experiments’?. I add brine water to the rotoflask and start to swing until all the oil stick in the glass… and can take out the water only to read the PH and put the nose there,smell a mixture of rancid and bubblegum( The oil in the flask smells like bubllegum). I forget to basific the pink water to see whats happen.
Then i add ether to the rotoflask and put in the Sfunnel and washing. Dry and done. I put in a mini jar add little heat and turn off. They finish dark in comparition with fresh frozen, but still have clarity. Maybe the acid prevent oxidation when this never happend. But for shit old and oxided material is not a miracle.i throw a nano pice of a dry oil that have in a sryngle to see the satursrion, now is start to sugar, i cap and i put a little warm.
Hmm interesting as heck @Vegeta
Vegeta…did it crystallize?
Ethyl acetate and pH’d anthocyanin was a hypothesis of mine for blue diamonds. Very interesting work!
Ohh Yes i forgot to share. I do hot cycling and start crash in 2 days, but i need to use the oil… so abort the test.
@vegeta, @mitokid. @Photon_noir
The ascorbic may be acting like a classic Hydrotrope.
With regards to THCA vs CBDA….(above)…of course
There is no harm in trying…but the solubility of THCA vs CBDA
In aprotic solvents is so different…especially when THCA is at
pH 2-3 …THCA exhibits some carboxylic acid Intramolecular or intermolecular bonding at low pH that shields the polar surface area . CBDA does not exhibit this phenomenon….most like likely due to the rescorcinolic OH (resonance or distributed pi system?)
I am sure Mito can comment in an intelligent manner.
So bottom line you can give it a try with CBDA…but do not get your expectations to high.
@Photon_noir …nice work up procedure for the H20 LLE wash…
I missed that post…when it first came out….
I’m going to look closely at it first chance I get.
Do you scrub the water out of alkane with sodium sulfate before the final crystallization step?
Hi moro, Yestsrday i am very active in reading, was follon sleep and the phone falls in my face and i touch something in this thread jjaa, you have a notification or something? Well No matter anaways. For the question, i always dry sodium sulfate before yes, only one time or two scrubing, always was a gentle agitation. In a month i Will harvest some Thc and i want to make a few test more in this matter,
Very interesting!
I can attest to the fact that you can separate CBDa with alkanes, KOH, and Citric Acid.
I dissolved a dab of winterized CO2 hemp oil (~1g) into heptane, loaded sep funnel and washed with pH 14 water. Drained water layer and repeated. Added pH 2 water (edit: to the water layer) until it went clear and it measured neutral (6.5). Oil crashed and floated to the top, recovered with heptane and evaporated on a rotovap to a nice purity. Don’t mind the CBD in the sample, I was in a hurry on the rotovap and removed the heptane at 45C.
And you notice that the " terp" fraction that remainds in the organic layer after the KHOwater smell really good?
I really want too know what’s happen with the other minors cannabinoids too.
Thanks for share this
Good test
Ok…
I should like to point out that at pH 6.5…
I don’t really understand “what” it is that crashes
Out. The cannabinoic acid should be ionized…
You’ve only titrated the phenolic OH.
I am assuming you mean added citric acid solution (pH=2),
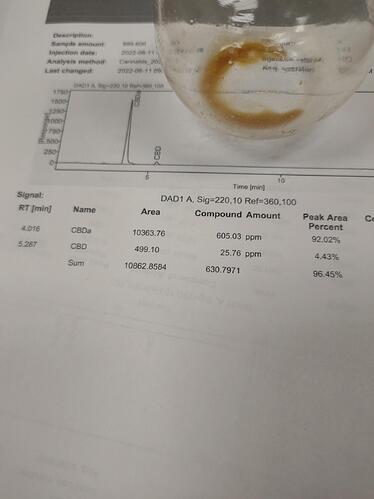
Until the pH=6.5. And what ever became insoluble was soluble by LLE into Heptane. And using HPLC methodology (mobile phase ?? We need data) the Heptane soluble material tested at 92% CBDA of total cannabinoid.
It is nice work in terms of purity and simplicity…
So when you have arrived at pH 6.5
We have K from KOH , citrate from citric acid and cannabinoate (COO-)
All in H20 solution.
how long did it take after you mixed the pH 2 citric acid…before precipitation?
Or did it oil- out as you added the acid?
Was the original “winterized CO@ hemp oil” redissolved in EtOH
To winterize, filtered and rotovaped? Or do you consider it CO2 winterized inline CO2 SCF ?
In other words, was your original material a concentrated EtOH solution?
Let me think a bit…
I know you have CBDA.
I am looking for a fast-crash, crystalline R-COOH cannabinoic acid.
If i not was dreaming i read some text that says that cbda can be salted like thca. Maybe is what it is… and lot of brains dont says nothing…
I feel like in general anything with appropriate pKa can be salted… I have precipitated CBDa and filtered it out. It was intensely utility dependent and made it not cost effective at all.
Doesn’t mean it isn’t possible.
You mean COO H acid form
Not salt ?
Did you get a amorphous crystalline form?
THCA and CBDA…exhibit considerably different solution chemistries.
Yes. Amorphous. If the material was already pretty pure then it would stack like a polycrystalline matrix.
I called it the clump. My boss patented it.
Can you post the patent?
Was it COOH form?
Composition of mother liquid and pH??
Would help if you don’t know for sure.
I’ve seen people laying out big trays of
Like white crystalline material…
Say “CBDA”.
But when I precipitate at pH 2 (HCl)
I don’t get pretty white crystals.
Crystallization is such an art form…I can readily admit ignorance of specifics.
Interesting topic! Thanks, @Vegeta, @moronnabis, @mitokid, @Photon_noir, etc.
But, I haven’t seen anyone mention potential butylated hydroxytoluene (BHT) contamination. I am interested in your thoughts on this issue.
Among other concerns, BHT is well-known to cause pulmonary toxicity from high dermal and ingestion doses. In a quick search, I only found one refence for pulmonary toxicity with limited data on inhaled BHT.
I, for one, wouldn’t want to dab BHT-contaminated concentrates, even if the BHT is present in minute amounts.
BHT is practically water-insoluble with a BP of 265°C.
As I’m sure you’re all aware, most diethyl ether is stabilized with BHT, an antioxidant to reduce ether peroxide formation. And dry ether peroxide crystals are contact/shock-sensitive explosives. Without BHT (or a similar stabilizer), ether peroxide crystals will form at the cap of older diethyl ether bottles. Historically, it wasn’t unheard of to cause a lab explosion when capping an old bottle of diethyl ether.
For anyone reading that isn’t aware:
If diethyl ether is prepared in the lab, purchased without BHT, or had BHT removed (e.g., a column of weak base ion exchange resin), to reduce peroxide formation, store in a cool, dark location under nitrogen or argon. @mitokid mentioned peroxide test strips; it’s a good idea to set up a testing schedule for older bottles (>1 year), or don’t store BHT-free diethyl ether for more than a year. And diethyl ether with BHT will still form peroxides, albeit at a slower rate, so proper storage is essential regardless.
Thanks for the heads up info on BHT.
I think only Vegeta mentioned the use of Ether early on.
I would not say it is in general usage… although one might see methyl-tertbutyl ether used in some research application.
For public consumption …ie rec market…I know Oregon doesn’’t allow it.
thanks you! dam…this really i did not know this. The ether i buy fresh and scrub with activated carbon and distill. But i will call to the company and ask if they uses BHT, this shit is everywer… food,cosmetics,solvents etc. ![]()