Apologies if this isn’t the correct place to ask about this, but I need some input from someone who has some knowledge on the subject and it’s sorta hard to come by, lol.
When D8 is heated and decarbed it produces 11-hydroxy, as does D9 which is why they have similar psychoactive effects. Am I correct for thinking this?
Looking to get some clarity on this as I’d like to be able to explain to people why D8 has the effects that it does.
Thanks!
11-oh-thc is not formed by heating and decarbing either d9 or d8 thc. Thc-a, when heated, will decarb and yield d9 thc.
11-oh-thc is formed after ingestion, in the body, by enzymatic activity.
okay, thank you for the clarification!
so how do i explain why d8 and d9 have similar effects? i know that it has a smaller chain so that explains why the effects are less intense.
and would a d8 edible change to 11-oh the same way a d9 edible would?
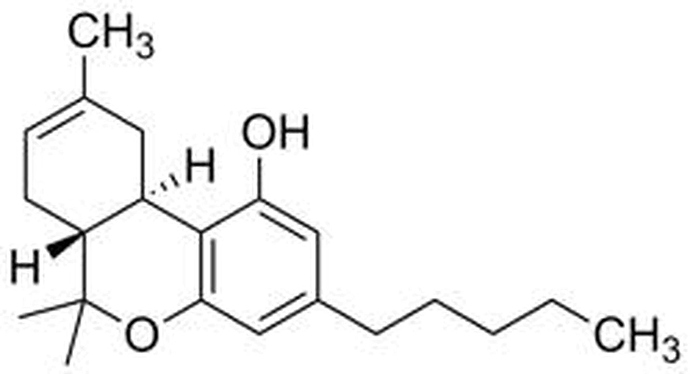
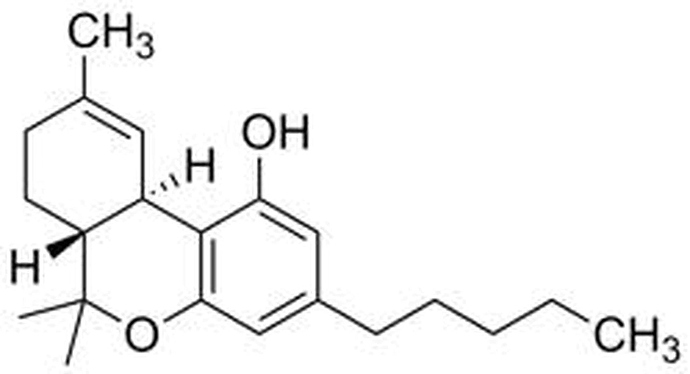
∆8-THC has the same configuration of atoms, the difference being the location of one double bond.
top image is ∆8-THC
second image is ∆9-THC
I can’t really speak much about the pharmacological differences between d8 and d9. The way I think about it, from a layperson’s perspective, the two are such close isomers of one another that they probably bind to and activate the same receptors as one another. Presumably d8 is a weaker agonist at the cb1 receptors due to it’s lessened psychotropic effect compared with d9.
Thc binds to and activates many more receptors than cb1 though. Being a weaker cb1 agonist than d9 probably doesn’t explain all of the differences between how the two compounds act in the body.
Okay, thank you for explaining that.
This was in response to someone claiming that when D8 is decarbed it becomes D9 and i was trying to disprove that because i just don’t see how that would be possible, as D9 oxidizes into D8.
I think thc oxidizes to form CBN. D9 thc thermally degrades to d8 thc. They both happen over time, and both processes are quickened by heat. I might be wrong about that though, probably best to double check my answer.
Heat alone wont produce D8 from d9 it’s needs to be heated with a catalyst to do that.
I thought I had read somewhere that d9 will degrade at higher temps into a bunch of stuff, d8 included. If not, my bad.
D8 is an isomer not a degradation product
I stand corrected then, thanks
I believe I read this also and it had to do with with CO2 extracted crude which could produce d8 when distilled.
CO2 + H2O forms carbonic acid.
Acid + heat => isomerization.