Good day all -
I have been a member of this forum for a minute now, mostly reading and occasionally chiming in. For the most part I’ve kept to the growing side of things as this is where my business and life is more focused.
Anyway, I’ve also spent a good deal of time in my life studying extraction and chemistry (in the field - not in university, that is) from a perspective of food science and nutritional products. I’ve had the opportunity to learn from and work with some amazing and gifted folk.
Recently, a mentor of mine came around and we began doing some experimental processing of some cbd-rich hemp using technique and material discovered and developed by this fellow. He has a western background in science and is a pHD, however he also has extensive training in a very ancient medicine path, natural/herbal/entheogenic plant medicine. To me he is the embodiment of a modern day alchemist. His insight into life below the level of the cell, elemental building blocks of life and their interactions, is quite deep.
So, through some trials and experimenting, we developed a method to extract the active element from the hemp and leave behind the carbon and chlorophyll. He refers to the method as plasma extraction. No chemicals or solvents are involved. Nor is it a method of mechanical separation. Using certain platinum group elements, those elements [importantly] being in an enzymatically active form, we were able to create an extract directly from fresh material which is high in CBD and also completely water soluble. Nothing added to the extract to create solubility. It just came out from the extraction as water soluble.
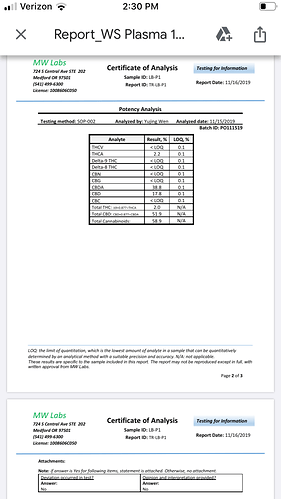
At first I wasn’t exactly sure what we’d done and guessed perhaps we’d actually left behind most of the oils in our extraction. However, initial lab tests have come back at 51.9% total CBD, with 38.8% CBDA and 17.8% CBD. Also 2.0% total THC.
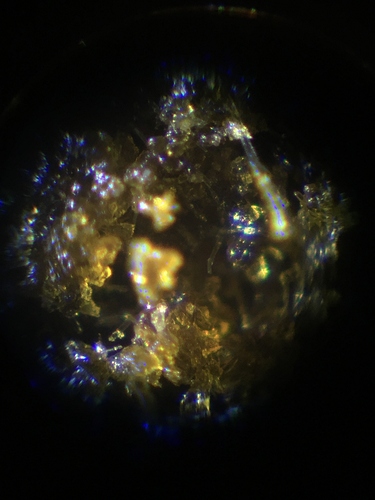
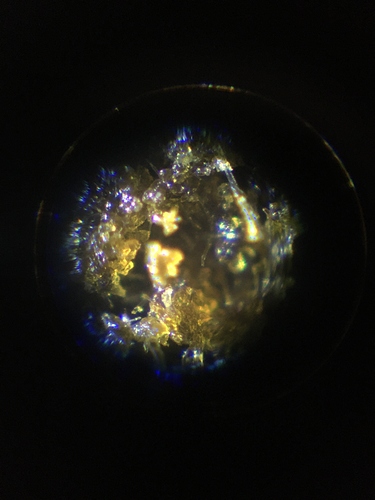
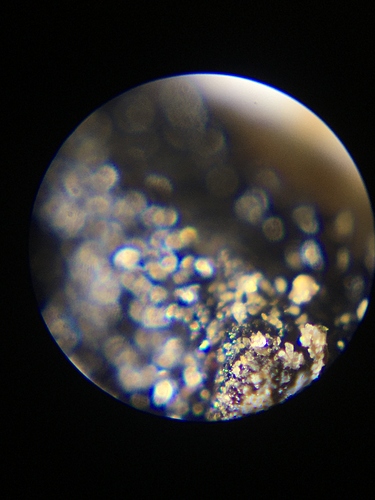
The color of it is dark, almost black, like old school afghani hash or something. The texture can be between completely dried out, brittle and ready to be brought down into a powder, to slightly gooey and stretchy depending on how much we dry it down. Under the microscope it looks really cool.
It dissolves very readily into water. The taste isn’t great but it’s waaaaay better than the taste of crude or something similar. In fact if you put it in water and add a bit of acid to it, it almost tastes neutral. Very easy to drink. The effects of it seem to onset very quickly.
I could see a whole range of applications for this material. Has anyone else played around to make a hemp/cannabis extract that retains it’s enzyme value from the fresh plant, in terms of bioavailability? I’m just wondering what this community thinks in general of this. Cheers.