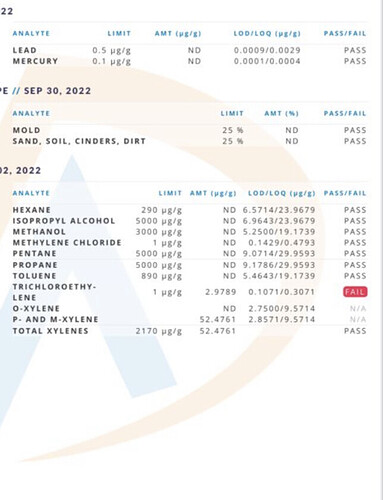
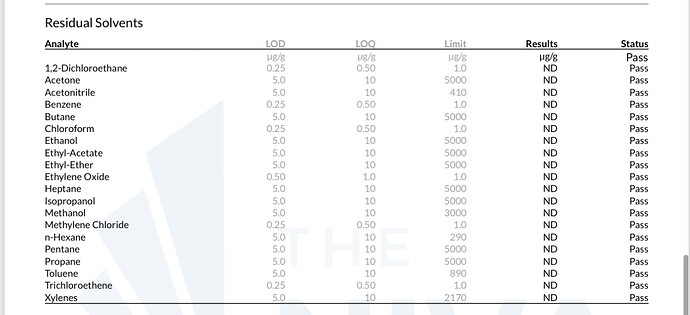
Just had a client complain about failed solvents in their finished product …
Never had an issue before
Wondering if testing lab chromatography … somehow effected the result
I think that’s from your gas
Second one is our test with ND in everything …
I’m just telling you where that “solvent” might have come from. You wouldn’t use that around the lab. It’s been mentioned around here before as the solvent they use to clean gas tanks and it may or may not be removed during gas distillation depending on how you do it.
As to why it is in one batch and not all: my guess is it was at the bottom or in the ball valve and it got sprayed out in a high enough concentration to detect on the first one and the rest shot clean.
Ask them for the chromatogram and do the check against the standards. If you are doing it in house, you probably have a similar standard. I first thought maybe your LOD was too high, but looks like you are saying your in-house validation method has LOD right around their LOQ so that’s probably not it.
Did you validate your in-house method - have you ever seen this before in-house, regardless of 3rd party testing? Is this the same lab you usually use and this is the first time they have this? Did you ask them for their internal investigation because of an out of trend result?
There is always possibility of contamination at testing, sampling, in your raw materials. Figuring out where is important as well. Contamination can happen in lots of ways - I don’t know where trichloroethylene contamination would come from in a normal production/lab setting… so I’d start with just making sure this is actually the peak they think it is and go from there.
Might be something simple - and it never hurts to ask. <3
what kind of product is this? Distillate, primary extract (shatter, sauce, rosin, … ), crystalline solid/isolate, natural/semi-synthetic/synthetic etc.
The more info known about how its processed the faster someone might be able to do root cause/CAPA.
Third party retest. May need to resample.
Umm—I would highly doubt there is trichloroethylene anywhere in your lab, even by accident.
Xylenes, I wouldn’t be super surprised if they were hiding in a liquid-at-room-temperature hydrocarbon solvent, but still highly unlikely.
Either 1) your extraction solvent was quite contaminated with these molecules 2) somewhere between extraction and testing these molecules were introduced 3) the lab that tested it is fuckin up in a tremendous fashion
3 is my guess
We had residual acetone in our samples last year only to find out they were actually terpenes causing the system to identify it as acetone. We don’t use acetone here so this caused me to ask for many retests which came back ND.
Thank you everyone for the solid and detailed answers …the finished product was distillate made in type 7 facility .
The lab reran the test and admitted that their machines were cleaned out that morning and there was a slight chance of contamination because they had 8 failed tests in a raw and everyone complained like we did ![]()
![]()
![]()
![]()
![]()
![]()
![]()
There it is
I thought we were going to battle the client …but it turned out ok in the end
This is the best outcome
Also the sad state of cannabis testing
Should have run validations after two results came back…having these sent to 8 clients is odd.
Those hits should have raised concerns earlier. At least have their PTs run by their states lab accreditation program.
Right and Out of spec or capa should have been triggered.
It’s early, had to go back and look that up. I’ll save anyone else a click.
Here’s a bonus pic of Johnny 5 as well:
Proper blanks and controls should have picked up this issue before it ever became one.
We recently published a paper on this. It is real, all an artifact of the headspace sampler. This would however not cause any chloro compounds to form. Xylenes maybe.
Ohhhh I’m so glad you wrote this article
Thank you
I actually sent it over to my client …