Hi everyone,
I’m working on a project and I’ve managed to make some “distillate” (don’t know what else to call it) that’s about 70% CBC…
Was wondering if anyone had any experience/tips with crystallizing CBC? What solvents/ratios do you use?
Any insights are appreciated.
How sure are you that its actually CBC and not a false identified peak?
Following the mantra of my PhD advisor: “It must be determined empirically”
You will just have to find out! Make a super saturated solvent solution and put it in the fridge or freezer for a week and see what happens!
2 Likes
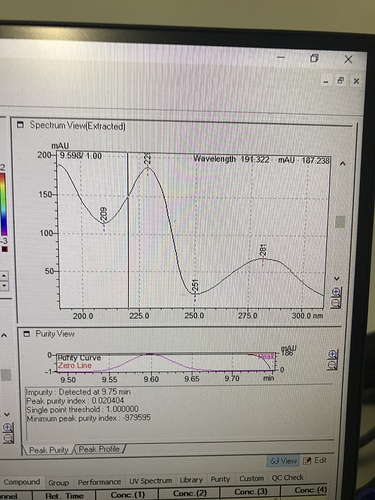
We matched the absorbance spectra for our CBC standard to this material using our photodiode-array detector on our HPLC. I am also confident it’s CBC because it’s such a high potency due to our initial chromatography. We don’t have a GS/MS or any NMR so a PDA is our most powerful tool.
So unless it’s a weird impurity that has absorbance spectra that looks exactly the same, I’m fairly convinced.
2 Likes
@thesk8nmidget
This is the spectra I was matching.
3 Likes
Awesome! Excited to see how the crystallization goes!
Best of luck and as @SoStupendous said just try a small amount and see what happens.
2 Likes
Can you post a chromatogram of the CBC as well? The absorbance profile is neat