Worth a shot! I tried something like this once and it didn’t work but that’s a way better setup then what I used.
Thanks for the feedback. Hopefully I can get the company to throw down for the lamp. If so, then I will post my results after everything clears with the state and I have lab results.
Uv lamp like what’s used in water systems?
Yup. Just like in water systems.
This may work! When I worked back in the pesticide field, one of the compounds we worked with was Spinosad. This molecule is quite photosensitive and we used to have studies that ended up with totes and totes of Spinosad contaminated water.
We would just recirculate the liquid from tote to tote with those submersible UV lights. We found that after X amount of recirculation time, everything had completely broken down and was completely unidentifiable via HPLC or LCMS. At this point it was a lot easier to dispose of the water as grey water.
So…I would say give it a shot! Just beware that excessive UV in this concentration may have a detrimental impact on the cannabinoids…but if it’s between removing all chlorfenapyr and potentially having a little degradation of cannabinoids it’s probably worth it.
My big question, is how much is the UV light going to degrade the cannabinoids to remediate to zero or ND on the pesticides?
TLDR: Don’t do it, please. <3
So I’m sitting over here with my quality hat on - and I know people do pesticide remediation, I’ve even done pesticide remediation.
But this stuff is not safe for humans. And the byproducts of the UV degradation (N-methoxyethylpyrrole-2-phenyl-3-cyano-4-Bromo-5-difluoromethylinum cation, N-methyl ethyl ether (2-trifluoromethyle-5-pyrrolium cation), 2(P-chlorophenyl)-3-carbonitrile pyrrolium cation and 2 (P-chlorophenyl)-3-cyano-4-oxo-5-trifluoromethyl pyrrolidine) are also nasty.
So you might “pass” a test for Chlorfenapyr - but what else are you then pushing into your customers?
Chlorfenapyr isn’t designed to be used on plants that will be consumed by humans OR animals.
So in this case - this is a bad idea because you won’t be removing the nasty from your product, you’ll be breaking it down into other nasties.
Gonna have to find a way to remove it without breaking it down into other terrible substances. I think you plan will work (there’s good evidence that it will degrade under UV) but I don’t think that will get you to a safe product for consumers.
If you mess this up - but still manage to pass testing… people could die. ![]() And if someone was ingesting your product over a longer period of time they could build this pesticide and its degradants up in their bodies - leading to long term exposure deaths.
And if someone was ingesting your product over a longer period of time they could build this pesticide and its degradants up in their bodies - leading to long term exposure deaths.
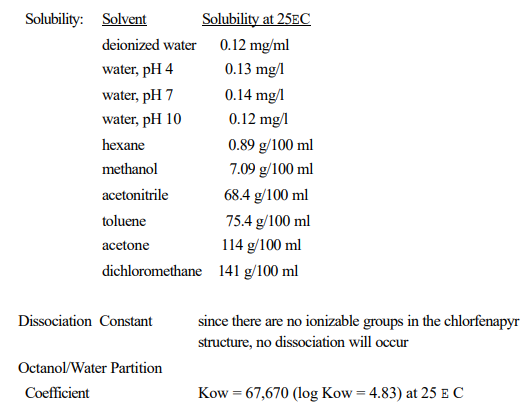
It seems to me that a liquid-liquid extraction and/or chromatography would be the safer option for the consumer. EPA has published the solubility information for this pesticide - the log Kow seems to be different enough to me that separation via some method should be possible.
There is a patent listed online for using Bentonite for chlorfenapyr removal. I haven’t ever sprayed this on my plants or at the plants of any of the companies I have worked for, so I haven’t tried this method. Maybe you could ask those guys - if this is actually effective and see if they have a specific mix/powder you can buy to do this work.
However, those breakdown products should be more easily remediable than the original chlorfenapyr was
Cool find! Does the package have anything resembling specs on the lamp?
Negative. I’m sure you could look it up online and find a user manual that has more detailed specs. These are generally used in ponds to keep algae at bay
I’m with you on this one. Pesticide remediation in general reminds me of the movie Wars Games.
In the end the final solution was the only way to win is not to play…
The point does still stand that if you assume it’s gone and/or don’t know what you’re looking for, you could be in a bad position
@ Cassin,
I appreciate your concern. I really do. But I feel there is nuance here.
The California standards for passing on pesticides are an order or two of magnitude more stringent than what other states require. They make their decisions based on a whole host of concerns beyond consumer safety. The environment and worker exposure are key among them. At this point, the grower already used or was inadvertently exposed to a banned pesticide. I can’t undo that. But what I can do is make sure the amount of the substance is well below what might pose a risk to the end consumer while also making money for my employer. If I believed I was genuinely placing people at risk I would not proceed.
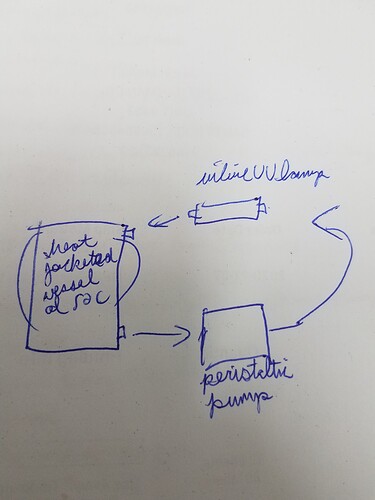
At this point I am going to recommend to my boss that we winterize the hot BHO and send it to distillation. If that doesn’t take care of it, I will run it through a column of magsil PR, do acid and base brine washes, and then set up the loop with the pump and UV light. I will post my results if and when I obtain them so stay tuned if this interests you.
So there are actually inline UV lamps out there that plug straight into triclamp. Gonna go with one of those.
If any one has any reading or experience on why even miniscule amounts of chlorfenapyr or its breakdown products are dangerous, please submit and I will take it under consideration.
It’ll also make your diary into CBN if you go easy bake oven route
Cannabinoids are not particularly soluble in ACN right?
Hexane and ACN might work- just tossing ideas out- you’d likely need to CPC it to get any process that can produce a volume worth the work
Also run that shit through T-5 see what happens- bentonite is amazing stuff